Abstract
OBJECTIVE
To assess the use of fMRI of the spinal cord in measuring noxious stimulation.
METHODS
The Scopus, Medline, EMBASE, and Web of Science databases were searched, along with the reference lists of included articles. Two independent reviewers screened abstracts, full-text articles, and extracted data. Original research was included if fMRI of the human spinal cord was used to measure responses to noxious stimulation.
RESULTS
Of the 192 abstracts screened, 19 met the search criteria and were divided according to their focus: investigating pain responses (n = 6), methodology (n = 6), spinal cord injury (n = 2), or cognition–pain interactions (n = 5). All but one study appear to have observed activity in ipsilateral and dorsal gray matter regions in response to noxious stimuli, although contralateral or ventral activity was also widely observed.
CONCLUSIONS
Although nociception can be investigated using spinal fMRI, establishing reliability, standardizing methodology, and reporting of results will greatly advance this field.
Keywords: pain, nociception, functional MRI, spinal cord, systematic review
Introduction
A substantial body of research has delineated the neural substrates of pain perception, both at the initial stage of peripheral sensory encoding (ie, nociception) and at later stages of perceptual and cognitive processing in the brain (ie, pain).1,2 The initial stage of nociception occurs at specialized pain receptors called nociceptors.3 Nociceptors innervate target tissue in muscles, joints, the digestive tract, and several internal organs, where they transduce noxious physical sensations into neural impulses that are transmitted along the cell’s axon to the dorsal horn of the spinal cord (see the study by Woolf and Ma4 for review). Numerous receptor types exist, including large diameter (1–5 μm), myelinated Aδ nociceptors that perform rapid transmission of thermal and mechanical stimulation, and small diameter (0.2–1.5 μm), unmyelinated C fibers, whose subtypes are sensitive to mechanical stimulation, heat, cold, and specific chemical irritants.5,6 Aδ- and C-fiber nociceptors provide afferent information to the dorsal horn of the spinal cord which, in turn, transmits ascending information to brainstem nuclei and the periaqueductal gray area.7 This initial input to the brain then projects to numerous cortical and subcortical regions, forming what is often referred to as a pain matrix,8 or cerebral signature.9 This network includes somatosensory cortical regions S1 and S2, the anterior cingulate cortex, the insula, and the prefrontal cortex.10 Neuroimaging researchers have suggested that this pain network could also be divided into two components, a lateral division, including S1 and S2, that analyzes the intensity and duration of pain, and a medial component involving the anterior cingulate cortex that is involved with the affective or evaluative response to the stimulus.11 Taken together, it is clear that researchers have reliable and well-established methods for detecting and quantifying activity at both the level of peripheral nociceptors and the level of subcortical12,13 and cortical brain regions.14–16 However, fewer research tools exist for measuring neural activity in the region of the nervous system that connects nociceptors to the brain—the spinal cord.
A cross-section of the spinal cord depicts a butterfly-shaped region of gray matter consisting of the bilateral dorsal horn and ventral horn (Fig. 1). The dorsal horn is typically involved with the processing of afferent sensory information, whereas the ventral horn houses neuronal cell bodies that innervate musculature and stimulate movement. These gray matter regions are surrounded by white matter (Fig. 1). Both Aδ- and C-fiber nociceptors transmit information from the periphery to neurons in the ipsilateral dorsal horn of the spinal cord.4 This region of the spinal cord is not uniform; rather, it is organized in a laminar fashion. Most nociceptive fibers synapse in layer I and in the dorsal half of layer II.17 A large proportion of the neurons found within these laminar layers have axons that remain within the spinal cord. These interneurons are either excitatory (releasing glutamate) or inhibitory (releasing gamma-aminobutyric acid or glycine). The activity of groups of interneurons can have (at least) two functions. First, they can influence reflexive responses to painful stimuli at the level of the spinal cord; this may involve interactions with ventral, motoric regions of the spinal cord. Second, they can modulate the neural signal transmitted to pain networks in the brain. This transmission occurs via projections from neurons in laminar layer I, which form tracts that modulate afferent input to the brain.1
Figure 1.
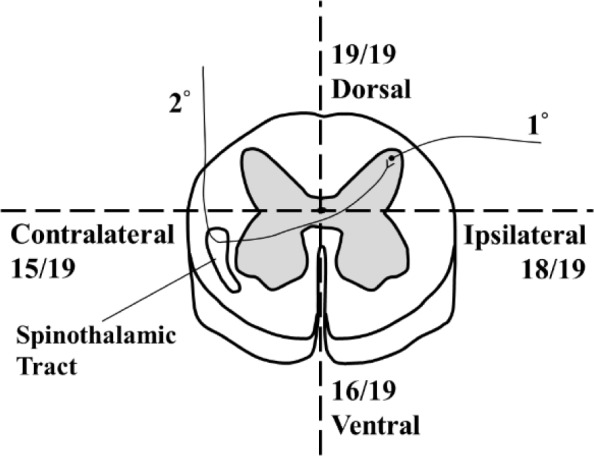
Schematic diagram of spinal cord physiology. 1° indicates primary afferent, while 2° indicates secondary neuron. Pain and temperature information travel the primary afferent, enter the spinal cord through the ipsilateral dorsal horn, and synapse with the secondary neuron. The secondary neuron decussates and transmits this information to the brain through the contralateral spinothalamic tract. Numbers (out of 19) indicate the number of studies in which activation in that region was observed.
The dorsal horn of the spinal cord is therefore a critical hub in the nervous system’s representation of pain.17 Indeed, aside from trigeminal nociceptors that innervate the face and head and project directly to the medulla, all pain responses in the body are processed by the dorsal horn.18 Therefore, spinal cord activity arising from noxious stimulation is expected in the ipsilateral dorsal horn (Fig. 1). This structure has also been implicated in numerous pain-related conditions.19 Unfortunately, much of our knowledge of the dorsal horn’s role in nociception comes from animal studies. Although informative, there is a clear clinical imperative to add neuroimaging studies of the human spinal cord (ie, spinal fMRI) to this existing knowledge base.
Spinal fMRI has made significant methodological advances in the past 20 years.20 It has gone from initial proof-of-principle studies detecting neural activity related to sensation and movement,21,22 to being used to examine much more nuanced research questions, such as emotion–movement interactions23,24 and autonomic nervous system activity.25 Its ability to detect activity in spinal cord regions related to pain could prove invaluable, both to our understanding of acute pain responses in healthy individuals and in chronic pain conditions. Indeed, spinal fMRI could be used to track changes in pain responses in degenerative conditions such as multiple sclerosis (MS).19 It may also prove useful in examining how descending modulation from the brain influences spinal cord responses to pain.26 Subjective pain responses—as shown by neuroimaging of the brain—can be modulated by attentional factors,27 anticipation,28 and mindfulness training.29 Using spinal fMRI to examine the impact of this modulation at the level of the spinal cord could provide important insights into how pain is experienced.
However, spinal fMRI is not without its challenges, largely arising from the spinal cord anatomy (for review, see the study by Stroman).30 Spinal fMRI requires a higher image resolution than cortical structures because of the small dimensions of the cord and the minute spatial differences between gray and white matter.30 The image resolution comes at the cost of the signal-to-noise ratio. The length of the cord makes it difficult to sample a large area transversely without drastically increasing the number of slices used. To combat this difficulty, some researchers have begun sampling the spinal cord in sagittal or coronal orientations. Sagittal and coronal slices increase the risk of partial volume effects; however, this can be overcome by smoothing in the rostral–caudal direction.30 An additional challenge associated with spinal fMRI is magnetic field inhomogeneity resulting from the different ways the bone, cartilage, and tissues distort the magnetic field.30 In contrast to imaging the brain, which remains relatively stationary, spinal cord imaging is complicated by various sources of motion artifacts from physiological noise (eg, ventilation, cardiac motion, and cerebrospinal fluid circulation).30 Additionally, conventional fMRI analysis software is created for brain imaging data and so does not include normalization tools for the spinal cord, and this limits the ability to run group-level analyses. As a result, statistical analysis methodology varies widely across spinal fMRI studies. Despite the challenges associated with acquiring and analyzing spinal fMRI data, a growing number of researchers have successfully imaged activity in the spinal cord in response to various study conditions and have produced results that correspond well with the known anatomy. A recent set of reviews outline the methodologies currently in use for overcoming these acquisition and analysis challenges.20,21
The purpose of the current research is to systematically summarize the existing spinal fMRI investigations of pain perception. Doing so serves two key functions. First, it will allow us to examine whether there is consistency across the existing spinal fMRI studies of pain. Second, it will show researchers which empirical questions have yet to be addressed and will hopefully serve as a catalyst for filling these holes in the literature.
Methods
Search strategy and selection criteria
In consultation with a medical librarian, we searched the Scopus, Medline, EMBASE, and Web of Science databases from inception to June 10, 2015. A comprehensive search strategy that incorporated Medical Subject Headings, text, and keywords was used to search for abstracts under three main themes: (1) functional magnetic resonance imaging; (2) spinal cords; and (3) pain. The reference lists of all included articles were reviewed to identify any further eligible studies. Studies were included if they reported on original research involving fMRI of the human spinal cord while a painful stimulus was applied. We included studies regardless of language or country of origin.
Study selection
Two reviewers (TAK and JK) independently reviewed all titles and abstracts, and all abstracts selected by either reviewer were retained. The same two reviewers independently screened all full-text articles for final inclusion, and any disagreements at this stage were resolved by consensus. Articles were included if they met the following criteria: (1) original research; (2) not solely an abstract; (3) reported on human fMRI findings; (4) in the spinal cord; and (5) with pain studied as an outcome.
Data extraction
Data were extracted from all included articles by two reviewers (TAK and JK) using a standardized form. Extracted information included the year of publication, number of participants, MRI sequence type, type of noxious stimuli, the stimulated dermatome and corresponding segment showing activation, and reported pain ratings. Demographic data included the sex distribution, mean or median age, handedness, and the location of data collection.
Data synthesis
Each study was classified according to stimulation type, including thermal, electrical, or mechanical pain manipulations. To aid in interpretation, the included studies were grouped into four categories based on the objective of the reported work: (1) investigating the use of spinal fMRI in experimental pain research; (2) advancing fMRI methodology using a pain paradigm; (3) examining nociception differences between spinal cord injured (SCI) patients and controls, and (4) exploring the relationship between pain and cognition. Activation patterns were described according to their anatomical location (ie, side of stimulation: ipsilateral or contralateral), segment, and dorsal or ventral horns.
Meta-analysis was not possible due to the considerable heterogeneity between studies.
Assessment of study consistency
Gaps in reporting the included studies were assessed qualitatively to identify areas for improved reporting in future research.
Results
Identification of studies
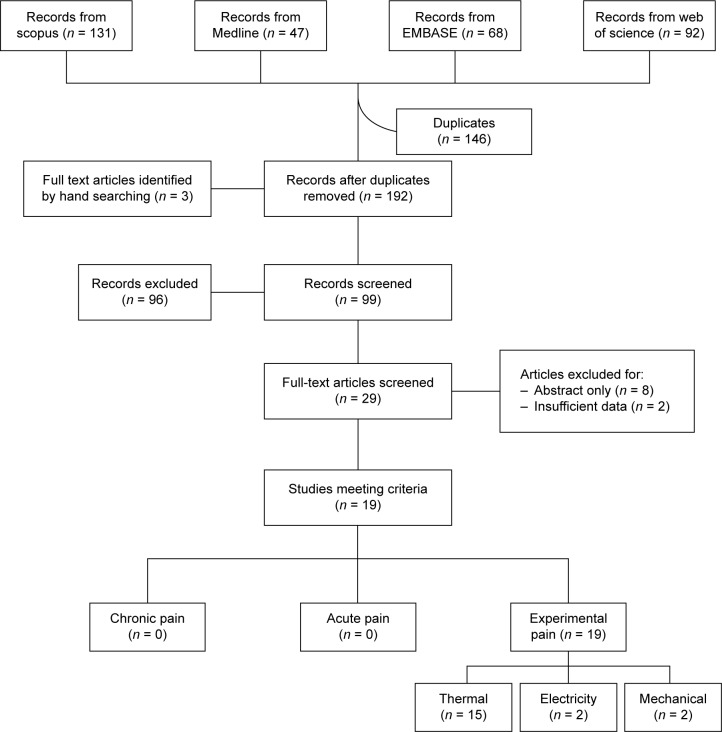
The search strategy yielded 338 total citations: 131 were found using Scopus, 47 from Medline, 68 from EMBASE, and 92 from Web of Science (Fig. 2). After duplicates were removed (146), there were 192 records to examine, as well as an additional three records identified by hand searching. From these records, 96 were excluded after reading the title and 99 abstracts were screened. Following this initial screening, 29 full-text articles were examined, after which 10 were removed (abstract only: eight; insufficient data: two), leaving 19 relevant articles. The articles excluded due to insufficient data were primarily focused on improving methodology for spinal cord fMRI, and although they used a pain paradigm, these data were not reported.
Figure 2.
Flowchart of procedure.
Details of included studies
Details of the included studies are in Table 1. Studies investigating pain in the spinal cord using fMRI ranged in year from 2002 to 2015. There does not appear to be any spinal fMRI data involving patients experiencing acute or chronic pain to date; thus, experimental pain was induced in all the included studies. The majority of the studies (n = 15)26,31–44 used thermal nociception, while electricity (n = 2)45,46 and mechanical (n = 2)47,48 methods were less common. Spin echo sequences were used in about half of the studies (n = 10),33,34,37,39,41,42,44,45,47,48 while the other half used a gradient echo sequence (n = 9).26,31,32,35,36,38,40,43,46 Additionally, activity was observed in both the cervical (n = 17)26,31–40,43–48 and lumbar (n = 2)41,42 regions of the spinal cord.
Table 1.
Characteristics of included studies.
| AUTHOR (YEAR) | POPULATION (N1, NX) | ECHO TYPE | NOXIOUS STIMULI (TIME (SEC)) | STIMULATED DERMATOME | PAIN RATING | FOCUS |
|---|---|---|---|---|---|---|
| Brooks (2008) | Norm (8, 10) | Gradient | Therm: 46.2°C; 46.1°C (30) | C8 | 5–6/10 | Methodology |
| Brooks (2012) | Norm (18) | Gradient | Therm: 52.3°C; 51.3°C (3) | C8 | 3.6/10 | Methodology |
| Cahill (2011) | Norm (8) | Spin | Therm: 42°C; 46°C (52) | ~C7a | 5.0/10 | Pain |
| Dobek (2014) | Norm (12) | Spin | Therm: 49.1°C, 10 pulses, 33 Hz (30) | C6 | 53/100; 57/100 | Cognition |
| Eippert (2009) | Norm (15) | Gradient | Therm: 46.9°C (20) | C6 | 60/100 | Cognition |
| Geuter (2013) | Norm (23) | Gradient | Therm: 46.3°C (20) | C5/C6 | 60/100 | Cognition |
| Ghazni (2010) | Norm (8) | Spin | Mech: 15 grams (56) | C5/C6 | 2.125/10 | Pain |
| Lawrence (2011) | Children (11) | Spin | Therm: 17°C; 27°C (43) | C6/C7 | Pain | |
| Nash (2013) | Norm (10) | Gradient | Therm: 47.5°C (30) | C6/C7; C4/C5 | 7/10 | Methodology |
| Rempe (2014) | Norm (16) | Spin | Mech: 166 mNb (40) | C6 | 3.4/10 | Pain |
| Rempe (2015) | Norm (16) | Spin | Therm: 36.8°Cb (34) | C6 | 4.5/10 | Pain |
| Sprenger (2012) | Norm (20, 15) | Gradient | Therm: 47.3°C (22.5) | C6 | 60/100 | Cognition |
| Sprenger (2015) | Norm (20) | Gradient | Therm: 46°C; 47°C (15) | C6 | 56.7/100 | Methodology |
| Stroman (2002) | SCI (6) Norm (15) | Spin | Therm: 10°C, 15°C, 2°C (33) | L4 | SCI | |
| Stroman (2004) | SCI (27) Norm (15) | Spin | Therm: 10°C (33) | L4 | SCI | |
| Stroman (2011) | Norm (11, 5, 9) | Spin | Therm: 18°C; 15°C (45) | ~C7a | 2.1/4; 2.3/4 | Cognition |
| Summers (2010) | Norm (11) | Gradient | Therm (Laser): 1.34 μm, 4 ms pulse, 0.45 Hz (20) | C6/C7 | 3–4/10 | Methodology |
| Xie (2007) | Norm (6) | Spin | Electr: 0.2 ms constant current, 20 Hz (35) | C6/C7a | Pain | |
| Xie (2012) | Norm (14) | Gradient | Electr: 1 ms pulse, 5 Hz (60) | C6a | 50–70/100 | Methodology |
Notes: Pain ratings were either averaged across participants, or the stimuli were individually adjusted to maintain a pain rating within a specified range. The values for the number of participants are separated by a comma if more than one protocol was used.
Dermatomes determined by hand from anatomical regions.
Sensitization via capsaicin.
Abbreviations: Norm, normal, healthy participants; Therm, thermal stimulation; Mech, mechanical stimulation; Electr, electrical stimulation; SCI, spinal cord injury.
Nine studies were conducted in Europe,26,31,32,35,36,39,40,43,48 nine in North America,33,34,37,38,41,42,44,46,47 and one in Asia.45 Mean participant age was reported in 15 studies that ranged from 8.8 to 41 years.26,32,33,35–44,46,48 One group reported a median age of 21.5 years.47 The number of participants ranged from 6 to 42 participants, although the study with 42 participants had 15 controls from previous work.42 Handedness was only reported in seven studies,32,33,39,43,45,47,48 and all but one of the participants were right handed.32 Participant sex was reported in all but one study,46 with ~39% being female and 61% being male.
The studies can be grouped into four basic categories according to their focus: (1) providing evidence that spinal fMRI can be performed for experimental pain conditions (n = 6),33,37,39,45,47,48 (2) improving methodology using nociception (n = 6),26,31,32,38,43,46 (3) comparing responses to nociception between SCI and neurologically healthy individuals (n = 2),41,42 and (4) extending spinal fMRI to investigate the interaction between nociception and cognition (n = 5).34–36,40,44 Regardless of the main objective of the study, similar activation patterns in response to painful stimuli are observed in all the studies; however, only a few researchers (n = 3) provide quantitative results, such as voxel counts, that would facilitate across-study comparisons and meta-analysis.31,46,47 One group reported x, y, and z coordinates for the most active voxel in each run and provided the number of voxels that comprised these clusters,38 but these coordinates are not entirely meaningful outside of the study as no standardized spinal cord map presently exists.
Activation patterns
Identification of spinal cord activity
Since spinal fMRI is a recently developed field, standard reporting guidelines do not yet exist. As a result, some data are more difficult to interpret. Reporting of the dermatome to which the stimulus was applied and/or the specific responses in the spinal cord segments is one such aspect. In 15 studies, the authors reported both pieces of information.26,31,32,34–43,47,48 In four cases,33,44–46 the authors reported the anatomical region to which the stimulus was applied—we inferred the dermatome that this region corresponded to. The specific spinal cord segment that showed activation was reported in only three of these studies.33,44,45 In one case, the authors did not report the dermatome stimulated or the specific segment showing activation, and instead indicated the activity at the vertebral level.46 All but one study reported the side of stimulus presentation and spinal cord activation.45 Eight studies had activity that was isolated to the dermatome stimulated,26,34–38,40,44 while the remaining 11 had either more diffuse activity, spanning multiple spinal cord segments, or activity within a few segments of expectation.31–33,39,41–43,45–48
Voxel counts were reported by meaningful spatial locations in only three of the 19 studies. In one study, voxel counts were reported per dorsal or ventral hemicord.31 In another study, voxel counts were reported per dorsal or ventral hemicord, and per spinal cord segment.46 In the last study, voxel counts were reported per segment.47 Coordinates of the most active voxel were reported in one study, but these coordinates are not easily interpretable or comparable to other studies as they would be with standardized brain coordinates.38 Unfortunately, statistical analyses were not performed on the voxel counts in any of these studies. Group or single-subject activation maps and qualitative statements regarding active voxel extent and location were reported in the remainder of the studies.26,32–45,48 However, without the authors providing a report of the quantification of the active voxels within each quadrant of each spinal cord segment, further analyses cannot be performed.
General trends
Although most spinal fMRI investigations of nociception reported results qualitatively, there are a number of data trends worth noting. All but one study reported or showed activity in activation maps in the side ipsilateral to stimulation; the anomalous study was unable to make such a determination due to their imaging parameters.46 Similarly, all studies reported or showed activity in the dorsal gray matter. Fifteen studies indicated that ipsilateral dorsal gray matter activity was the greatest or only significant signal change observed in the spinal cord in response to pain.26,31,33–42,44,45,48 One study indicated that both ipsilateral dorsal gray matter and contralateral ventral gray matter were active and did not indicate if either were significantly more active.47 Another study indicated that ipsilateral activity was greater than contralateral activity, but dorsal activity did not appear to be greater than ventral activity.32
Although the literature frequently reports ipsilateral dorsal activity, the spread of activation also extends into contralateral and ventral regions. Contralateral activity was observed in 15 studies, but this activity was often minimal and generally less than in ipsilateral regions.32–39,41–45,47,48 Additionally, ventral activity was also observed in 16 studies.31,32,34–39,41–48 However, this ventral and contralateral activity is often minimal and is seen in nonpain conditions involving cognitive tasks,34 in short stimulation blocks (possibly indicating a reflex response),32 in a placebo only condition,35 and for mechanical stimuli evoking a low pain rating.47 Only three studies clearly demonstrated ipsilateral dorsal horn activity without much ventral or contralateral activity.26,35,40 Unfortunately, activity was not consistently localized to the ipsilateral dorsal quadrant at the subject level.
Pain and methodology
In two-thirds of the included studies, researchers were focused primarily on determining if results from fMRI corresponded to the known physiology of pain transmission in the spinal cord, or improving methodology to better detect this activity. Activity in these papers was generally consistent with physiology but the focus of the research was more concerned with optimizing imaging parameters and controlling factors such as physiological noise and cord motion. For example, in one study, researchers controlled for physiological noise and cerebrospinal fluid motion, concluding that controlling for these factors drastically reduced the signal in the spinal cord, likely reducing the false positive rate.32 Upon controlling for physiological and CSF noise, the researchers observed ipsilateral activity that was not localized to either the ventral or dorsal hemicord.32 Reviews have already been completed on improved methodology for spinal fMRI.20,49
Pain and spinal cord injury
SCI patients were examined using spinal fMRI in two of the studies.41,42 The patient group consisted of complete and incomplete SCI, and all participants were exposed to noxious stimuli at the L4 dermatome that was below the site of injury. Patients with incomplete SCI who had spared sensory function produced activation similar to that of the neurologically intact controls (ie, ipsilateral dorsal horn, extending to central and ventral regions, and contralateral ventral regions). Patients with complete SCI showed diminished activity in the ipsilateral dorsal gray matter but showed increased activity in the ipsi- and contralateral ventral gray matter compared to controls. Incomplete SCI in which the patients were unable to feel the noxious stimuli produced attenuated activity in the ipsilateral dorsal gray matter, but unlike the complete injured patients showed decreased ipsi-and contralateral activity in the ventral gray matter compared to controls. It is interesting to note that although the same peripheral stimulus was being applied, patients who could not feel the noxious stimulus showed decreased ipsilateral dorsal horn activity. These results suggest that spinal fMRI is sensitive enough to differentiate between nociceptive processing in SCI patients and neurologically healthy controls; such differences may reflect changes in descending modulation from the brain. However, given the small sample size in these studies and the variability in the SCI classifications, further studies are required before definitive claims can be made.
Pain–cognition interactions
Building on studies that have shown that neural responses to pain can be detected in the spinal cord, research has begun to investigate whether nociceptive responses in the spinal cord can be modulated by top–down processing in the brain. In two studies, the effects of psychogenic responses to pain using either a placebo to reduce subjective pain,35 or a nocebo to increase subjective pain are considered.36 Participants in the placebo study were convinced that lidocaine cream was applied to a small region on their left forearm, while a control cream was applied to another. Both creams were inert and heat was applied to each region in separate runs. Interestingly, activation in the ipsilateral dorsal gray matter was reduced and contralateral ventral activity was increased in response to the placebo treatment, at the level of the spinal cord (uncorrected for multiple comparisons).35 A similar study design was used for the nocebo treatment,36 but instead of the lidocaine cream, an inert cream labeled as capsaicin—the ingredient that makes chili peppers spicy—was used. Again, cognition was used to manipulate both subjective pain and physiological responses; increased activity was observed throughout the C5/C6 spinal cord segment—with the peak voxel in the ipsilateral hemicord—with greater activation occurring due to the nocebo than to a pain condition using the same temperature.36 These results indicate that the brain can modulate pain responses at the level of the spinal cord.
Further evidence of descending pain modulation was observed in one study where the analgesic effects of listening to music during noxious stimulation were investigated.34 Participants reported lower subjective pain ratings while their favorite music was played, and showed decreased activity in the ipsilateral dorsal horn and increased activity in the contralateral ventral horn.34 These results indicate that a reduction of subjective pain—a construct of the brain—is associated with attenuated spinal cord activity in nociceptive pathways.
Unfortunately, the cognition–pain interaction in the spinal cord is complicated when the focus is shifted to attention. Two studies manipulated participants’ attention while they were exposed to noxious stimuli;40,44 interestingly, the results differed entirely. In one experiment, participants’ attention was diverted from the pain using a 1-back and 2-back working memory task, after which they rated the subjective pain.40 The 1-back task produced activity in the ipsilateral dorsal gray matter, as did the control pain condition (uncorrected for multiple comparisons).40 However, the 2-back attentional task resulted in no significant spinal cord activation. A significant reduction in activity was observed in ipsilateral dorsal horn between the 2-back and 1-back conditions.40 In the other experiment, attention was diverted using three different methods: detecting the number of new speaking characters in a movie, determining the direction of coherently moving dots among distractors, and answering mentally challenging questions.44 Surprisingly, during a rating task in which participants were focused on the noxious stimulus being applied, activity in the ipsilateral dorsal gray matter was negative, but was positive during the attentionally demanding tasks.44 However, a few possible explanations were provided. Participants rated the thermal stimulation during the attention tasks as more painful than during the rating task, perhaps because the ratings for the cognitive task were performed retrospectively, or because the thermal stimuli were distracting or annoying and thus perceived with a greater intensity.44 Additionally, this study used stimuli that elicited mild discomfort with a rating of 2.1 or 2.3/4, while others had pain ratings as high as 7/10 (Table 1).
Study consistency
Various methodological differences exist in the body of literature on responses to painful stimuli using spinal fMRI, complicating the interpretation of the data. The studies differed in terms of fMRI data acquisition: some used gradient echo and some used spin echo sequences (Table 1), some interpreted results in terms of blood-oxygenation-level-dependent (BOLD) signal changes,26,31,32,34–36,38,40,43,46 others referred to signal enhancement by extravascular water protons (SEEP) signal changes,41,42,44,45,47 and some report spin echo sequence imaging parameters that are optimized for measuring both.33,37,39,48 Field strength was either 1.5 T31,41,42,45 or 3 T;26,32–35,37–40,43,44,46–48 one group did not mention the field strength.36 Directionality of image acquisition also differed: some images were obtained in a sagittal direction,33–35,37,39,44,46–48 while others were obtained axially,26,31,32,36,38,40–43,45 resulting in differing ranges of spatial extent of spinal cord covered in each study.
In addition to inconsistencies in data acquisition, differences in the study paradigms exist. The duration of the noxious stimulation paradigms differed, ranging from 3 to 60 seconds (Table 1). Inconsistencies in the reporting of the subjective experience of the painful stimulation were also present. Most researchers used a visual analog scale for pain or a similar scale for discomfort to report their pain ratings;26,31–36,38–40,43,44,46–48 however, some did not report subjective pain ratings at all and instead simply stated that the stimuli were noxious.37,41,42,45 Although all papers include which anatomical region was stimulated, some did not clearly translate this anatomical location into a dermatome (n = 4)33,44–46 or state the corresponding spinal cord segment (n = 1).46 A reader knowledgeable in spinal cord physiology can determine this anatomy. A potential reason for this lack of detail regarding the stimulation paradigms is that in some cases the researchers were more interested in a different, methodological research question and simply used painful stimuli as a means to this end.
The image preprocessing and analysis software also differed: custom-written MATLAB code,33,34,37,44,47 SPM (various versions),26,35,36,38,40 IDL,41,42 FSL,31,32,43 and AFNI43,45 were all used. Two studies did not report the software used.39,48 Various statistical methodologies were employed to produce the imaging results, and the resulting spinal fMRI maps were presented at differing thresholds and with26,32,44,46 or without corrections for multiple comparisons.31,33–43,45,47,48 Although methodology has been developed allowing researchers to group imaging data across subjects, and to compare between study groups or conditions,50,51 in a few of the papers imaging data are presented on a subject-by-subject basis.43,45 Other researchers reported group values but only include single-subject maps.31,46 The absence of statistics demonstrating the reproducibility of these individual-level results and the variability in reporting of the group-level results makes it difficult to compare the studies and interpret this body of literature.
Discussion
This systematic review was concerned with determining to what extent fMRI can be used to assess nociception in the spinal cord. Although we included in our search the possibility of locating spinal fMRI papers on acute or chronic pain, no such papers were found. Given the logistical difficulties of imaging nonexperimental acute pain, and due to the lack of reproducible resting state spinal fMRI studies in healthy volunteers with which to compare changes in spinal cord physiology in chronic pain conditions, the paucity of such papers is not surprising. The results trend toward the known anatomy of the spinal cord; activation in the ipsilateral dorsal gray matter seems to be associated with painful stimulation.17 However, many of the results presented were not corrected for multiple comparisons, were primarily reported qualitatively, were not analyzed at the group level, or did not assess individual-level reproducibility. Unfortunately, no studies specifically tested the reliability of spinal fMRI in localizing activity at any level. Therefore, although the results are intuitively appealing, we must be cautious in our interpretation of the data.
Unfortunately, due to the heterogeneity of the reporting of results, meta-analysis was not possible; ultimately, mathematical quantification of the results would be ideal. The results typically reported are qualitative and descriptive in nature; however, there is no reason why quantitative data—voxel counts and/or percent signal changes with corresponding statistical thresholds—cannot be reported. A consistent reporting of the location of stimulus presentation along with a quantification of activity in each quadrant of each spinal cord segment is required. In addition to the commonly reported positive signal changes, the less often reported negative signal changes are also important. These data could provide further insight into spinal cord baseline activity and the interaction with other neurons, both within the spinal cord circuitry and the brain’s descending modulation. Reporting only positive activation has the potential to greatly oversimplify complex physiological responses. A meta-analysis of this information would have allowed us to establish between-group reliability, presently lacking for spinal fMRI.
Moving forward, reliability and reproducibility need to be established within and between subjects. This would greatly advance the legitimacy of moving spinal fMRI into the clinical realm. Because it has been shown that the spinal cord physiology and functionality can change over time due to injury,42 future studies may survey chronic pain patients at different points in time to determine whether the presence of chronic pain is altering the spinal cord. Spinal fMRI may also prove helpful in characterizing demyelinating disease states such as MS.19 Since injury and disease states affecting the spinal cord (eg, MS, SCI) often have comorbidity with affective disorders,52,53 future research may investigate the interactions between pain and affect. As indicated in this review, it is possible to measure cognition–pain interactions, and research has investigated the role of the spinal cord in emotion independently.23–25 However, thus far, the interaction between affect and pain has not been directly studied, and cognition–pain interactions require further investigation.
Conclusions
The high level of inconsistency in methodology and statistical analyses makes interpretation of spinal fMRI investigations of nociception difficult. The lack of quantified results makes meta-analysis impossible for these spinal fMRI studies, and this limits the claims that can be made for reliability in this literature. Although the results of the studies cannot be directly compared, in general, there appears to be a response in the ipsilateral dorsal horn of the spinal cord when noxious stimuli are applied, as expected based on anatomical findings. Before spinal fMRI can be used in a clinical setting, for either diagnosis or measuring potential treatments, the reliability at the individual level needs to be established. Future research should develop scanning protocols to measure chronic pain in order to determine how spinal cord responses differ from those of the healthy population and from experimentally induced pain. In order for spinal fMRI to reach its research and clinical potential, full reporting of methodology and results along with assessed reliability is required.
Acknowledgments
The authors wish to thank Tania Gottschalk for her assistance with the literature search.
Footnotes
ACADEMIC EDITOR: Sendhil Velan, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 3,136 words, excluding any confidential comments to the academic editor.
FUNDING: The authors would like to acknowledge the support of the Health Sciences Centre Foundation (JK) and the Natural Sciences and Engineering Research Council of Canada (SDS). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the study: TAK, KMF, and JK. Analyzed the data: TAK and JK. Wrote the first draft of the manuscript: TAK, KMF, SDS, and JK. Contributed to the writing of the manuscript: TAK, KMF, SDS, and JK. Agreed with manuscript results and conclusions: JK. Jointly developed the structure and arguments for the paper: TAK, KMF, SDS, and JK. Made critical revisions and approved the final version: TAK, KMF, SDS, and JK. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat. 2005;207(1):19–33. doi: 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garland EL. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care. 2012;39(3):561–571. doi: 10.1016/j.pop.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherrington CS. Observations on the scratch-reflex in the spinal dog. J Physiol. 1906;34(1–2):1–50. doi: 10.1113/jphysiol.1906.sp001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf CJ, Ma Q. Nociceptors—noxious stimulus detectors. Neuron. 2007;55(3):353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh DJ, Lee H, Lo L, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106(22):9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 7.Gauriau C, Bernard JF. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol. 2004;468(1):24–56. doi: 10.1002/cne.10873. [DOI] [PubMed] [Google Scholar]
- 8.Ingvar M. Pain and functional imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1347–1358. doi: 10.1098/rstb.1999.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracey I. Imaging pain. Br J Anaesth. 2008;101(1):32–39. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- 10.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 12.Tracey I, Iannetti GD. Brainstem functional imaging in humans. Suppl Clin Neurophysiol. 2006;58:52–67. doi: 10.1016/s1567-424x(09)70059-5. [DOI] [PubMed] [Google Scholar]
- 13.Tracey I, Ploghaus A, Gati JS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22(7):2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71(2):802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- 15.Jones AK, Friston K, Frackowiak RS. Localization of responses to pain in human cerebral cortex. Science. 1992;255(5041):215–216. doi: 10.1126/science.1553549. [DOI] [PubMed] [Google Scholar]
- 16.Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251(4999):1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- 17.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren K, Dubner R. Central nervous system plasticity and persistent pain. J Orofac Pain. 1999;13(3):155–163. discussion 164–171. [PubMed] [Google Scholar]
- 19.Wheeler-Kingshott CA, Stroman PW, Schwab JM, et al. The current state-of-the-art of spinal cord imaging: applications. Neuroimage. 2014;84:1082–1093. doi: 10.1016/j.neuroimage.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroman PW, Wheeler-Kingshott C, Bacon M, et al. The current state-of-the-art of spinal cord imaging: methods. Neuroimage. 2014;84:1070–1081. doi: 10.1016/j.neuroimage.2013.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroman PW, Ryner LN. Functional MRI of motor and sensory activation in the human spinal cord. Magn Reson Imaging. 2001;19(1):27–32. doi: 10.1016/s0730-725x(01)00226-0. [DOI] [PubMed] [Google Scholar]
- 22.Yoshizawa T, Nose T, Moore GJ, Sillerud LO. Functional magnetic resonance imaging of motor activation in the human cervical spinal cord. Neuroimage. 1996;4(3 pt 1):174–182. doi: 10.1006/nimg.1996.0068. [DOI] [PubMed] [Google Scholar]
- 23.McIver TA, Kornelsen J, Smith SD. Limb-specific emotional modulation of cervical spinal cord neurons. Cogn Affect Behav Neurosci. 2013;13(3):464–472. doi: 10.3758/s13415-013-0154-x. [DOI] [PubMed] [Google Scholar]
- 24.Smith SD, Kornelsen J. Emotion-dependent responses in spinal cord neurons: a spinal fMRI study. Neuroimage. 2011;58(1):269–274. doi: 10.1016/j.neuroimage.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Kornelsen J, Smith SD, McIver TA. A neural correlate of visceral emotional responses: evidence from fMRI of the thoracic spinal cord. Soc Cogn Affect Neurosci. 2015;10(4):584–588. doi: 10.1093/scan/nsu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprenger C, Finsterbusch J, Buchel C. Spinal cord-midbrain functional connectivity is related to perceived pain intensity: a combined spino-cortical fMRI study. J Neurosci. 2015;35(10):4248–4257. doi: 10.1523/JNEUROSCI.4897-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85(1–2):19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 28.Ploghaus A, Narain C, Beckmann CF, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21(24):9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCracken LM Gauntlett-Gilbert J, KE Vowles. The role of mindfulness in a contextual cognitive-behavioral analysis of chronic pain-related suffering and disability. Pain. 2007;131(1–2):63–69. doi: 10.1016/j.pain.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Stroman PW. Magnetic resonance imaging of neuronal function in the spinal cord: spinal FMRI. Clin Med Res. 2005;3(3):146–156. doi: 10.3121/cmr.3.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks JC, Beckmann CF, Miller KL, et al. Physiological noise modelling for spinal functional magnetic resonance imaging studies. Neuroimage. 2008;39(2):680–692. doi: 10.1016/j.neuroimage.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Brooks JC, Kong Y, Lee MC, et al. Stimulus site and modality dependence of functional activity within the human spinal cord. J Neurosci. 2012;32(18):6231–6239. doi: 10.1523/JNEUROSCI.2543-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cahill CM, Stroman PW. Mapping of neural activity produced by thermal pain in the healthy human spinal cord and brain stem: a functional magnetic resonance imaging study. Magn Reson Imaging. 2011;29(3):342–352. doi: 10.1016/j.mri.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Dobek CE, Beynon ME, Bosma RL, Stroman PW. Music modulation of pain perception and pain-related activity in the brain, brain stem, and spinal cord: a functional magnetic resonance imaging study. J Pain. 2014;15(10):1057–1068. doi: 10.1016/j.jpain.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326(5951):404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 36.Geuter S, Buchel C. Facilitation of pain in the human spinal cord by nocebo treatment. J Neurosci. 2013;33(34):13784–13790. doi: 10.1523/JNEUROSCI.2191-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence JM, Kornelsen J, Stroman PW. Noninvasive observation of cervical spinal cord activity in children by functional MRI during cold thermal stimulation. Magn Reson Imaging. 2011;29(6):813–818. doi: 10.1016/j.mri.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Nash P, Wiley K, Brown J, et al. Functional magnetic resonance imaging identifies somatotopic organization of nociception in the human spinal cord. Pain. 2013;154(6):776–781. doi: 10.1016/j.pain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Rempe T, Wolff S, Riedel C, et al. Spinal and supraspinal processing of thermal stimuli: an fMRI study. J Magn Reson Imaging. 2015;41(4):1046–1055. doi: 10.1002/jmri.24627. [DOI] [PubMed] [Google Scholar]
- 40.Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Curr Biol. 2012;22(11):1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Stroman PW, Tomanek B, Krause V, Frankenstein UN, Malisza KL. Mapping of neuronal function in the healthy and injured human spinal cord with spinal fMRI. Neuroimage. 2002;17(4):1854–1860. doi: 10.1006/nimg.2002.1305. [DOI] [PubMed] [Google Scholar]
- 42.Stroman PW, Kornelsen J, Bergman A, et al. Noninvasive assessment of the injured human spinal cord by means of functional magnetic resonance imaging. Spinal Cord. 2004;42(2):59–66. doi: 10.1038/sj.sc.3101559. [DOI] [PubMed] [Google Scholar]
- 43.Summers PE, Ferraro D, Duzzi D, Lui F, Iannetti GD, Porro CA. A quantitative comparison of BOLD fMRI responses to noxious and innocuous stimuli in the human spinal cord. Neuroimage. 2010;50(4):1408–1415. doi: 10.1016/j.neuroimage.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 44.Stroman PW, Coe BC, Munoz DP. Influence of attention focus on neural activity in the human spinal cord during thermal sensory stimulation. Magn Reson Imaging. 2011;29(1):9–18. doi: 10.1016/j.mri.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Xie CH, Kong KM, Guan JT, Chen YX, Wu RH. Functional MR imaging of the cervical spinal cord by use of 20 Hz functional electrical stimulation to median nerve. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:3392–3395. doi: 10.1109/IEMBS.2007.4353059. [DOI] [PubMed] [Google Scholar]
- 46.Xie G, Piché M, Khoshnejad M, et al. Reduction of physiological noise with independent component analysis improves the detection of nociceptive responses with fMRI of the human spinal cord. Neuroimage. 2012;63(1):245–252. doi: 10.1016/j.neuroimage.2012.06.057. [DOI] [PubMed] [Google Scholar]
- 47.Ghazni NF, Cahill CM, Stroman PW. Tactile sensory and pain networks in the human spinal cord and brain stem mapped by means of functional MR imaging. AJNR Am J Neuroradiol. 2010;31(4):661–667. doi: 10.3174/ajnr.A1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rempe T, Wolff S, Riedel C, et al. Spinal fMRI reveals decreased descending inhibition during secondary mechanical hyperalgesia. PLoS One. 2014;9(11):e112325–e112325. doi: 10.1371/journal.pone.0112325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Figley CR, Leitch JK, Stroman PW. In contrast to BOLD: signal enhancement by extravascular water protons as an alternative mechanism of endogenous fMRI signal change. Magn Reson Imaging. 2010;28(8):1234–1243. doi: 10.1016/j.mri.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Bosma RL, Stroman PW. Assessment of data acquisition parameters, and analysis techniques for noise reduction in spinal cord fMRI data. Magn Reson Imaging. 2014;32(5):473–481. doi: 10.1016/j.mri.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Leitch JK, Figley CR, Stroman PW. Applying functional MRI to the spinal cord and brainstem. Magn Reson Imaging. 2010;28(8):1225–1233. doi: 10.1016/j.mri.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 52.Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862–1868. doi: 10.1176/appi.ajp.159.11.1862. [DOI] [PubMed] [Google Scholar]
- 53.Fann JR, Bombardier CH, Richards JS, et al. PRISMS Investigators. Depression after spinal cord injury: comorbidities, mental health service use, and adequacy of treatment. Arch Phys Med Rehabil. 2011;92(3):352–360. doi: 10.1016/j.apmr.2010.05.016. [DOI] [PubMed] [Google Scholar]