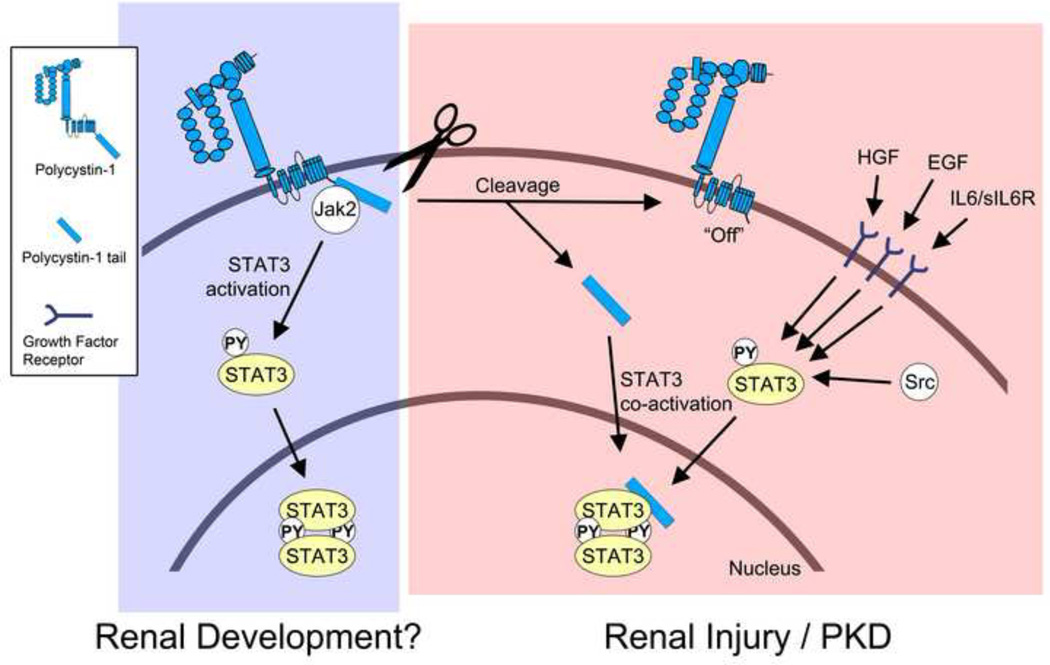
Figure. Schematic of current model of STAT3 signaling in the normal and diseased kidney.
During renal development, membrane-anchored, full-length PC1 may cause direct activation of STAT3 via JAK2 that is associated with its C-terminal cytoplasmic tail. Direct STAT3 activation by PC1 would be an intrinsic pathway that is independent of growth factor-mediated STAT3 signaling. It is currently unknown how the activation of STAT3 by full-length PC1 is regulated. It is possible that an - yet unidentified - extracellular ligand may trigger STAT3 activation, or that the extracellular domain of PC1 engages in homo-typic interactions. It is also possible that fluid flow may regulate this activity. During renal injury and in PKD PC1 appears to undergo proteolytic cleavage that releases its cytoplasmic tail into the cytoplasm. This turns “off” the ability of the remaining membrane-anchored portion of PC1 to activate STAT3. However, the soluble PC1 tail can now translocate to the nucleus and co-activate STAT3 that has been activated by prior growth factor signaling. In addition to STAT3, the cleaved PC1 tail can also co-activate STAT1 and STAT6 (not shown here). Therefore, the cleaved PC1 tail may have the ability to amplify different signaling pathways that lead to different cellular responses depending on the growth factor and cytokine environment.