Abstract
Objective
Acute otitis externa (AOE) is a common but preventable ear condition. Clinical guidelines issued in 2006 recommended topical treatments for uncomplicated AOE, but systemic antimicrobials appear to be commonly prescribed. The objective of this analysis was to describe pre- and postguideline prescribing patterns by clinician specialty and antimicrobial type and assess trends over time.
Study Design
Retrospective longitudinal analysis of a large insurance database.
Setting
Outpatient departments in the United States.
Methods
Initial outpatient visits in 2004 to 2010 for AOE (excluding visits with complicating conditions) were extracted from an insurance database. Prescription drug claims were linked and categorized by clinician specialty and antimicrobial type.
Results
The analysis included 907,261 initial outpatient visits. Use of systemic antimicrobials declined by 4.9% (95% confidence interval [CI], 4.1%, 5.7%) from 36.5% of initial visits in 2004 to 32.1% in 2010. Use of systemic antimicrobials varied by specialty. Systemic antimicrobials were prescribed in 47.1% of 2010 emergency department (ED) visits (−6.9% from 2004, 95% CI –12.3, −1.5), 25.9% of otolaryngologist visits (−1.6%, 95% CI –5.6, 2.4), and 20.4% of pediatrician visits (−6.6%, 95% CI –8.8, –4.4). Penicillins were prescribed most frequently (42.3% of systemic prescriptions in 2010), followed by cephalosporins (19.8%), erythromycin/macrolides (17.4%), and quinolones (11.1%). Opioids were prescribed in 26.4% of ED visits and 9% of outpatient visits.
Conclusions
Use of systemic antimicrobials declined over time, but one-third of 2010 visits resulted in systemic antimicrobials, despite exclusion of visits with complicating factors. Use of systemic antimicrobials varied by specialty. Further educational efforts and outreach to other specialties might be warranted.
Keywords: otitis externa, outpatient visits, antibiotics, antimicrobial resistance, opioids, prescribing patterns
Acute otitis externa (AOE), the common but preventable inflammation of the outer ear known as “swimmer’s ear,”1,2 leads to approximately 2.4 million ambulatory and emergency department (ED) visits each year, taking up more than half a million hours of providers’ time annually.3 Prevention strategies focus on keeping the ears dry (ie, drying the ear after swimming, bathing, or showering using alcohol-based drops or a hair dryer), while the accessible nature of the externa ear canal suggests that AOE is well-suited to topical treatment.
Evidence-based clinical practice guidelines published in 2006 call for initial treatment of uncomplicated AOE with topical antimicrobial preparations, including preparations containing antibiotics, steroids, or low-pH antiseptics (eg, aluminum acetate).4,5 Systemic antimicrobial treatment has not shown additional benefits for uncomplicated AOE5 and is recommended only when complicating factors are present (eg, diabetes or conditions predisposing patients to wider infection). Topical treatments are formulated for activity against Pseudomonas aeruginosa and Staphylococcus aureus (the most common organisms implicated in AOE) and, unlike systemic treatments, can deliver doses of antimicrobial ingredients well above the minimum inhibitory concentration of resistant organisms.6
Analyses conducted in the United States prior to release of the current clinical guidelines suggested high rates of systemic antimicrobial use, with systemic antimicrobials prescribed in one-quarter to one-half of visits.7,8 A comparison of systemic antimicrobial use before and after guideline publication found little change in prescribing patterns, suggesting low levels of adoption and adherence to the guidelines by clinicians.9 Description of pre- and postguideline prescribing patterns by clinician specialty or antimicrobial type could identify opportunities to increase awareness of the evidence-based guidelines.
Methods
Outpatient visits (ambulatory care and ED visits) for AOE and prescriptions were assessed using insurance claims from 2004 to 2010 (the most recent year for which data were available) from the Marketscan Commercial Claims and Encounters database. The database contains deidentified, preexisting insurance billing records. Because no interaction or intervention with human subjects occurred and no personally identifiable information was used, collected, or transmitted in the course of this analysis of previously collected data, the analysis was not considered human subjects research (as defined in 45 CFR part 46) subject to review by the institutional review board. The database contains a large convenience sample of commercially insured persons. There was some variation in participating employers and health plans over the study period. Sample size increased each year, from 13 million covered lives in 2004 to 45 million covered lives in 2010.
Initial outpatient visits for AOE (ICD-9-CM codes 380.10 and 380.12) with a prescription filled within 1 day and without a concurrent diagnosis of otitis media (OM); diabetes; cellulitis, periauriculitis, or lymphadenitis; immune compromise (as defined in the list of immunocompromising conditions in the Prevention Quality Indicators Technical Specifications published by the Agency for Health Research and Quality10); or malignant otitis externa were included. Initial visits were defined as AOE visits for patients who did not have an AOE visit within the past 365 days. There were 1,155,169 AOE visits with a prescription filled within 1 day. Of those visits, 140,384 visits (12.2%) were follow-up visits, 113,553 (9.8%) had a concurrent diagnosis of otitis media, 5,245 (0.5%) had a concurrent diagnosis of cellulitis or other evidence of wider infection, 4919 (0.4%) had a concurrent diagnosis of diabetes, 174 (0.0%) had a concurrent immunocompromising condition, and 19 (0.0%) had a concurrent diagnosis of malignant otitis externa. A total of 247,908 visits (21.5%) were excluded because they had 1 or more of these exclusionary factors.
Prescription drug claims were categorized by clinician specialty and antimicrobial type. To assess trends in prescribing patterns over time for each specialty, Poisson regression was used. Poisson regression is used to model count data and can be adapted to model proportions or rates through the use of an offset (ie, modeling counts per unit of a denominator variable, such as person-time, instead of modeling count data alone). PROC GENMOD and PROC NLMIXED (SAS version 9.2, Cary, North Carolina) were used, and data were modeled using the number of visits resulting in a prescription of the medication type of interest as the dependent variable, the year as the independent variable, and the total number of visits as the offset. The proportion of visits resulting in a prescription of the medication type of interest and Wald 95% confidence intervals (CIs) for the proportion were calculated using estimate statements. CIs for the total change in percentage of visits resulting in a prescription of the medication type of interest from 2004 to 2010 were calculated as described in SAS documentation for calculation of rate differences and CIs using PROC NLMIXED.11 The types of systemic antimicrobials prescribed by each clinical specialty in 2010 (the most recent year for which complete data were available) were assessed using univariate Poisson regression, with the number of visits resulting in a prescription of the systemic antimicrobial drug of interest as the dependent variable, the specialty as the independent variable, and the total number of visits resulting in a systemic antimicrobial prescription as the offset.
Results
The analysis included 907,261 initial outpatient visits. Most visits were to ambulatory care, while 7.5% were ED visits. For outpatient records, up to 4 ICD-9-CM codes can be reported for each visit. The most frequently co-occurring diagnostic codes were otalgia (2.6%) and impacted cerumen (2.4%).
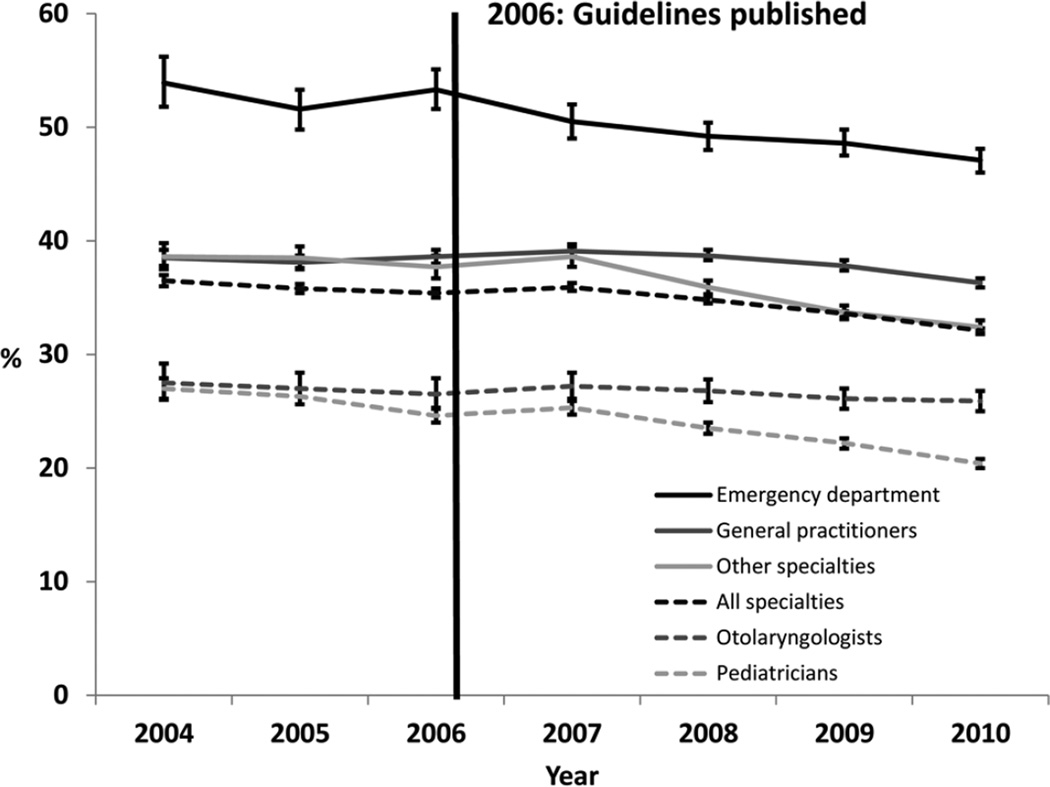
Overall, use of systemic antimicrobials declined from 36.5% of initial visits in 2004 to 32.1% in 2010 (−4.4%, 95% CI −5.0, −3.8; Table 1, Figure 1). Use of systemic antimicrobials declined significantly in each specialty studied, with the exception of otolaryngologists (27.5% of visits in 2004, 25.9% of visits in 2010 [−1.6%, 95% CI −5.6, 2.4]). The largest decline occurred among ED physicians (53.9% of visits in 2004 to 47.1% of visits in 2010 [−6.9%, 95% CI −12.3, −1.5]). Visits in which only topical antimicrobials were prescribed showed a concurrent rise, from 60.4% in 2004 to 65.3% in 2010 (4.9%, 95% CI 4.1, 5.7). Pediatricians (78.3% of visits in 2010) and otolaryngologists (65.5% of visits in 2010) were most likely to prescribe topical antimicrobial treatments only.
Table 1.
Prescribing Patterns for Initial Outpatient Visits with a Diagnosis of Acute Otitis Externa, by Clinician Specialty, Marketscan Commercial Claims and Encounters Database, United States, 2004–2010
| Percentage of Visits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Specialty | 2004 (n = 60,188) |
2005 (n = 89,932) |
2006 (n = 89,708) |
2007 (n = 117,452) |
2008 (n = 160,226) |
2009 (n = 183,497) |
2010 (n = 206,258) |
Percentage Change, 2004–2010a |
| All specialties (n = 907,261) | ||||||||
| Topical onlyb | 60.4 | 61.3 | 61.7 | 61.1 | 62.2 | 63.6 | 65.3 | 4.9 (4.1, 5.7) |
| Any systemicb | 36.5 | 35.8 | 35.4 | 35.9 | 34.8 | 33.6 | 32.1 | −4.4 (−5.0, −3.8) |
| Opioid pain reliever | 9.0 | 8.8 | 8.9 | 9.1 | 8.4 | 7.8 | 7.3 | −1.7 (−2.0, −1.4) |
| NSAID pain reliever | 1.7 | 1.7 | 1.6 | 1.7 | 2.1 | 2.0 | 1.9 | 0.2 (0.0, 0.3) |
| Otolaryngologists (n = 57,567) | ||||||||
| Topical onlyb | 64.2 | 64.5 | 64.7 | 64.4 | 64.5 | 65.1 | 65.5 | 1.3 (−4.9, 7.5) |
| Any systemicb | 27.5 | 27.0 | 26.5 | 27.2 | 26.8 | 26.1 | 25.9 | −1.6 (−5.6, 2.4) |
| Opioid pain reliever | 9.5 | 10.1 | 9.1 | 10.0 | 9.6 | 8.2 | 8.9 | −0.6 (−3.0, 1.7) |
| NSAID pain reliever | 1.3 | 1.0 | 1.1 | 1.1 | 1.0 | 1.2 | 1.3 | 0.0 (−0.8, 0.9) |
| Pediatricians (n = 203,639) | ||||||||
| Topical onlyb | 71.3 | 72.3 | 74.0 | 73.3 | 75.1 | 76.4 | 78.3 | 7.0 (3.3, 10.7) |
| Any systemicb | 27.0 | 26.3 | 24.6 | 25.3 | 23.5 | 22.2 | 20.4 | −6.6 (−8.8, −4.4) |
| Opioid pain reliever | 3.3 | 3.0 | 2.9 | 2.7 | 2.2 | 2.1 | 2.0 | −1.3 (−2.0, −0.5) |
| NSAID pain reliever | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | 0.4 | 0.4 | 0.2 (−0.0, 0.4) |
| General practitioners (n = 399,974) | ||||||||
| Topical onlyb | 58.7 | 59.0 | 58.6 | 57.9 | 58.4 | 59.4 | 61.0 | 2.3 (0.1, 4.5) |
| Any systemicb | 38.5 | 38.1 | 38.6 | 39.1 | 38.7 | 37.8 | 36.3 | −2.2 (−4.0, −0.4) |
| Opioid pain reliever | 8.2 | 8.0 | 8.1 | 8.6 | 7.8 | 7.4 | 6.9 | −1.3 (−2.1, −0.5) |
| NSAID pain reliever | 2.1 | 2.1 | 1.9 | 2.1 | 2.5 | 2.5 | 2.3 | 0.2 (−0.2, 0.6) |
| Emergency department (n = 67,837) | ||||||||
| Topical onlyb | 40.2 | 43.3 | 42.0 | 44.6 | 46.2 | 47.2 | 48.7 | 8.5 (3.7, 13.4) |
| Any systemicb | 53.9 | 51.6 | 53.3 | 50.5 | 49.2 | 48.6 | 47.1 | −6.9 (−12.3, −1.5) |
| Opioid pain reliever | 35.4 | 33.8 | 33.8 | 32.7 | 29.9 | 28.3 | 26.4 | −9.1 (−13.4, −4.7) |
| NSAID pain reliever | 4.5 | 5.0 | 5.1 | 4.5 | 5.6 | 5.4 | 5.1 | 0.6 (−1.0, 2.2) |
| Other specialtiesc (n = 178,244) | ||||||||
| Topical onlyb | 58.6 | 59.1 | 60.0 | 58.9 | 61.8 | 64.1 | 65.7 | 7.0 (3.4, 10.7) |
| Any systemicb | 38.6 | 38.5 | 37.7 | 38.6 | 35.9 | 33.7 | 32.4 | −6.2 (−9.1, −3.3) |
| Opioid pain reliever | 7.5 | 8.1 | 8.5 | 8.6 | 7.7 | 7.1 | 6.5 | −1.0 (−2.3, 0.3) |
| NSAID pain reliever | 1.7 | 1.7 | 1.4 | 1.8 | 2.2 | 1.9 | 1.7 | 0.1 (−0.6, 0.7) |
Abbreviation: NSAID, nonsteroidal anti-inflammatory drug.
Percentage change: the difference and 95% confidence interval for percentage of visits resulting in prescription for the specified medication type from 2004 to 2010, calculated using Poisson regression.
The “topical only” and “any systemic” categories do not sum to 100 because a small proportion of visits received neither category of medication.
Other specialties: category includes specialties not classified elsewhere (eg, members of a multispecialty physician group or health care providers at an urgent care facility).
Figure 1.
Prescribing patterns for systemic antimicrobials by clinician specialty, Marketscan Commercial Claims and Encounters database, United States, 2004 to 2010. Error bars indicate 95% confidence intervals for the percentage of visits resulting in a systemic antibiotic prescription, calculated using Poisson regression.
Prescriptions for opioid pain relievers declined significantly from 2004 (9.0% of outpatient visits and 35.4% of ED visits) to 2010 (7.3% of outpatient visits [−1.7%, 95% CI −2.0, −1.4) and 26.4% of ED visits (−9.1%, 95% CI −13.4, −4.7). Only 1.9% of 2010 outpatient visits resulted in a non-steroidal anti-inflammatory drug (NSAID) prescription, a proportion that did not significantly increase from 2004 (0.2% increase, 95% CI 0.0, 0.3).
When systemic antimicrobials were prescribed, penicillins were prescribed most frequently (42.3% in 2010), followed by cephalosporins (19.8%), erythromycin/macrolides (17.4%), and quinolones (11.1%; Table 2). Overall, prescriptions for penicillins (−3.6%, 95% CI −4.7, −2.4) and cephalosporins (−4.0%, 95% CI −4.8, −3.1) decreased, while prescriptions for erythromycin/macrolides (2.2%, 95% CI 1.5, 2.9) and sulfonamides (4.6%, 95% CI 4.2, 4.9) increased.
Table 2.
Prescribing Patterns for Initial Outpatient Visits for Acute Otitis Externa where Systemic Antimicrobials Were Prescribed, by Clinician Specialty, Marketscan Commercial Claims and Encounters Database, United States, 2004–2010
| Percent of Visitsa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Specialty | 2004 (n = 21,948) |
2005 (n = 32,193) |
2006 (n = 31,743) |
2007 (n = 42,182) |
2008 (n = 55,774) |
2009 (n = 61,564) |
2010 (n = 66,137) |
Percentage Changeb |
| All specialties (n = 311,541) | ||||||||
| Penicillin | 45.8 | 45.9 | 44.0 | 42.0 | 42.2 | 41.2 | 42.3 | −3.6 (−4.7, −2.4) |
| Cephalosporin | 23.7 | 22.6 | 23.1 | 22.2 | 20.4 | 20.3 | 19.8 | −4.0 (−4.8, −3.1) |
| Macrolide | 15.2 | 15.8 | 15.4 | 15.9 | 17.0 | 17.0 | 17.4 | 2.2 (1.5, 2.9) |
| Quinolone | 11.2 | 11.3 | 12.0 | 12.6 | 12.2 | 12.1 | 11.1 | −0.1 (−0.6, 0.5) |
| Sulfonamide | 2.3 | 2.9 | 3.7 | 5.0 | 5.9 | 6.7 | 6.8 | 4.6 (4.2, 4.9) |
| Antifungal | 1.5 | 1.3 | 1.4 | 1.7 | 1.7 | 2.0 | 2.0 | 0.5 (0.3, 0.7) |
| Tetracycline | 1.1 | 1.1 | 1.3 | 1.6 | 1.6 | 1.8 | 1.9 | 0.8 (0.6, 1.0) |
| Other | 0.5 | 0.6 | 0.7 | 1.2 | 1.4 | 1.5 | 1.5 | 1.0 (0.8, 1.1) |
| Otolaryngologists (n = 15,278) | ||||||||
| Penicillin | 29.3 | 27.3 | 29.0 | 25.5 | 28.8 | 27.1 | 26.6 | −2.7 (−10.7, 5.2) |
| Cephalosporin | 20.3 | 21.3 | 21.6 | 22.1 | 17.7 | 18.2 | 19.7 | −0.6 (−7.3, 6.0) |
| Macrolide | 9.7 | 10.5 | 8.6 | 8.4 | 9.3 | 9.6 | 9.1 | −0.5 (−5.1, 4.0) |
| Quinolone | 32.2 | 34.6 | 33.6 | 34.6 | 34.0 | 32.6 | 32.2 | 0.0 (−8.4, 8.5) |
| Sulfonamide | 1.7 | 3.0 | 3.2 | 3.9 | 5.3 | 6.0 | 6.0 | 4.4 (1.9, 6.9) |
| Antifungal | 5.4 | 2.9 | 3.4 | 3.9 | 3.9 | 4.5 | 3.7 | −1.7 (−5.0, 1.6) |
| Tetracycline | 2.1 | 1.7 | 1.2 | 1.1 | 1.3 | 1.8 | 1.9 | −0.2 (−2.3, 2.0) |
| Other | 1.6 | 1.1 | 1.8 | 2.5 | 2.0 | 2.4 | 3.3 | 1.7 (−0.4, 3.8) |
| Pediatricians (n = 47,560) | ||||||||
| Penicillin | 61.1 | 60.1 | 59.5 | 55.6 | 53.7 | 53.9 | 53.8 | −7.4 (−14.0, −0.7) |
| Cephalosporin | 23.9 | 24.1 | 25.5 | 28.5 | 28.0 | 27.1 | 27.2 | 3.3 (−1.0, 7.6) |
| Macrolide | 11.7 | 12.4 | 11.0 | 11.4 | 12.2 | 12.8 | 12.2 | 0.5 (−2.5, 3.5) |
| Quinolone | 0.5 | 1.1 | 0.9 | 1.0 | 1.1 | 0.9 | 1.1 | 0.5 (−0.2, 1.3) |
| Sulfonamide | 1.9 | 2.0 | 2.5 | 2.6 | 3.8 | 3.8 | 4.2 | 2.4 (1.0, 3.7) |
| Antifungal | 0.4 | 0.4 | 0.4 | 0.5 | 0.3 | 0.5 | 0.7 | 0.4 (−0.2, 1.0) |
| Tetracycline | 0.7 | 0.4 | 0.3 | 0.3 | 0.5 | 0.6 | 0.6 | −0.1 (−0.8, 0.6) |
| Other | 0.3 | 0.1 | 0.5 | 1.0 | 1.2 | 1.2 | 1.2 | 1.0 (0.3, 1.6) |
| General practitioners (n = 151,982) | ||||||||
| Penicillin | 43.2 | 42.3 | 40.2 | 39.1 | 39.3 | 38.4 | 39.6 | −3.6 (−6.6, −0.5) |
| Cephalosporin | 23.6 | 22.9 | 23.2 | 21.5 | 19.5 | 19.9 | 18.9 | −4.7 (−7.0, −2.5) |
| Macrolide | 16.7 | 17.3 | 17.4 | 18.1 | 19.0 | 19.0 | 19.9 | 3.2 (1.2, 5.1) |
| Quinolone | 12.3 | 12.6 | 13.6 | 14.4 | 13.7 | 13.3 | 12.1 | −0.1 (−1.8, 1.5) |
| Sulfonamide | 2.3 | 2.9 | 3.4 | 4.4 | 5.6 | 6.5 | 6.4 | 4.1 (3.2, 5.0) |
| Antifungal | 1.7 | 1.5 | 1.8 | 1.9 | 2.3 | 2.4 | 2.5 | 0.8 (0.2, 1.5) |
| Tetracycline | 1.3 | 1.3 | 1.6 | 2.0 | 2.0 | 2.2 | 2.3 | 1.0 (0.4, 1.6) |
| Other | 0.4 | 0.4 | 0.5 | 0.8 | 1.0 | 1.0 | 1.0 | 0.6 (0.3, 1.0) |
| Emergency department (n = 33,692) | ||||||||
| Penicillin | 46.7 | 47.0 | 45.6 | 44.9 | 44.1 | 42.8 | 43.1 | −3.6 (−10.6, 3.4) |
| Cephalosporin | 25.3 | 21.8 | 20.7 | 17.5 | 17.4 | 17.5 | 17.4 | −7.8 (−12.8, −2.8) |
| Macrolide | 13.5 | 14.0 | 14.0 | 13.8 | 14.1 | 14.1 | 14.3 | 0.8 (−3.1, 4.6) |
| Quinolone | 10.9 | 12.1 | 12.3 | 12.6 | 12.2 | 12.9 | 11.9 | 1.0 (−2.4, 4.5) |
| Sulfonamide | 3.3 | 4.2 | 6.9 | 10.4 | 10.9 | 11.5 | 12.7 | 9.5 (7.1, 11.9) |
| Antifungal | 0.6 | 0.8 | 0.6 | 0.8 | 0.5 | 0.9 | 0.9 | 0.3 (−0.5, 1.2) |
| Tetracycline | 0.7 | 1.0 | 1.2 | 1.6 | 1.5 | 1.7 | 1.9 | 1.2 (0.2, 2.2) |
| Other | 1.0 | 2.0 | 1.7 | 2.8 | 3.3 | 3.6 | 3.7 | 2.7 (1.4, 4.0) |
| Other specialties (n = 63,029)c | ||||||||
| Penicillin | 44.8 | 46.5 | 43.3 | 41.1 | 42.4 | 41.4 | 43.4 | −1.3 (−6.5, 3.8) |
| Cephalosporin | 24.1 | 21.1 | 22.6 | 21.3 | 19.0 | 18.5 | 17.9 | −6.2 (−9.8, −2.5) |
| Macrolide | 16.3 | 17.2 | 16.9 | 17.1 | 18.9 | 18.9 | 18.8 | 2.5 (−0.7, 5.7) |
| Quinolone | 11.1 | 10.6 | 11.5 | 11.7 | 11.9 | 11.6 | 10.4 | −0.7 (−3.2, 1.9) |
| Sulfonamide | 2.2 | 3.2 | 4.1 | 6.5 | 5.6 | 6.7 | 6.7 | 4.5 (3.1, 5.9) |
| Antifungal | 1.4 | 1.3 | 1.3 | 1.8 | 1.7 | 2.1 | 2.0 | 0.6 (−0.4, 1.5) |
| Tetracycline | 0.9 | 1.1 | 1.2 | 1.6 | 1.6 | 1.9 | 2.1 | 1.2 (0.3, 2.0) |
| Other | 0.6 | 0.6 | 0.7 | 1.5 | 1.4 | 1.5 | 1.3 | 0.7 (0.0, 1.4) |
Percentages do not sum to 100 because 2.2% of visits where systemic antimicrobials were prescribed resulted in multiple systemic antimicrobial prescriptions.
Percentage change: the difference and 95% confidence interval for percentage of visits resulting in a prescription of the specified systemic antibiotic from 2004 to 2010, calculated using Poisson regression.
Other specialties: category includes specialties not classified elsewhere (eg, members of a multispecialty physician group or health care providers at an urgent care facility).
In 2010, the most recent year for which complete data were available, otolaryngologists prescribed a greater proportion of quinolones when systemic antimicrobials were prescribed (32.2% of visits, 95% CI 30.3, 34.2) and a smaller proportion of penicillins (26.6%, 95% CI 24.9, 28.5) than other specialties (Table 3). Pediatricians prescribed penicillins 53.8% of the time (95% CI 52.3, 55.2) and quinolones in 1.1% of visits (95% CI 0.9, 1.3).
Table 3.
Type of Systemic Antimicrobials Prescribed in Initial Outpatient Visits for Acute Otitis Externa Where Systemic Antimicrobials Were Prescribed (n = 66,137), by Clinician Specialty, Marketscan Commercial Claims and Encounters Database, United States, 2010
| Otolaryngologists (n = 3168) |
Pediatricians (n = 9628) |
General Practitioners (n = 31,853) |
Emergency Department (n = 7436) |
Other Specialtiesa (n = 14,052) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial Type | %b | 95% CI | %b | 95% CI | %b | 95% CI | %b | 95% CI | %b | 95% CI |
| Penicillin | 26.6 | (24.9, 28.5) | 53.8 | (52.3, 55.2) | 39.6 | (38.9, 40.3) | 43.1 | (41.6, 44.6) | 43.4 | (42.4, 44.5) |
| Cephalosporin | 19.7 | (18.2, 21.3) | 27.2 | (26.1, 28.2) | 18.9 | (18.5, 19.4) | 17.4 | (16.5, 18.4) | 17.9 | (17.2, 18.6) |
| Macrolide | 9.1 | (8.1, 10.2) | 12.2 | (11.6, 13.0) | 19.9 | (19.4, 20.4) | 14.3 | (13.4, 15.1) | 18.8 | (18.1, 19.5) |
| Quinolone | 32.2 | (30.3, 34.2) | 1.1 | (0.9, 1.3) | 12.1 | (11.7, 12.5) | 11.9 | (11.1, 12.7) | 10.4 | (9.9, 11.0) |
| Sulfonamide | 6.0 | (5.2, 6.9) | 4.2 | (3.8, 4.7) | 6.4 | (6.1, 6.7) | 12.7 | (11.9, 13.6) | 6.7 | (6.3, 7.1) |
| Antifungal | 3.7 | (3.1, 4.4) | 0.7 | (0.6, 0.9) | 2.5 | (2.4, 2.7) | 0.9 | (0.7, 1.2) | 2.0 | (1.8, 2.3) |
| Tetracycline | 1.9 | (1.5, 2.5) | 0.6 | (0.4, 0.7) | 2.3 | (2.1, 2.5) | 1.9 | (1.6, 2.2) | 2.1 | (1.9, 2.4) |
| Other | 3.3 | (2.7, 4.0) | 1.2 | (1.0, 1.5) | 1.0 | (0.9, 1.1) | 3.7 | (3.3, 4.2) | 1.3 | (1.1, 1.5) |
Other specialties: category includes specialties not classified elsewhere (eg, members of a multispecialty physician group or health care providers at an urgent care facility).
%: percentage of visits in which systemic antimicrobials were prescribed. Percentages do not sum to 100 because 2.8% of visits in which systemic antimicrobials were prescribed resulted in multiple systemic antimicrobial prescriptions.
Discussion
More than 900,000 pre- and postguideline outpatient visits for AOE were included in this detailed analysis of prescribing patterns. The frequency of systemic antimicrobial prescriptions showed a decline from 2004 to 2010 within each clinical specialty studied. However, declines were modest (−4.4% overall [95% CI −5.0, −3.8], from 36.5% to 32.1%), and one-third of visits in 2010 resulted in prescriptions for systemic antimicrobials, despite exclusion of repeat visits and visits with complicating factors. The use of systemic antimicrobials varied by specialty. Otolaryngologists and pediatricians had the lowest rate of systemic antimicrobial use overall, while ED physicians were most likely to prescribe systemic antimicrobials.
An estimated 2.4 million outpatient visits for AOE without a concurrent diagnosis of OM occurred in 2007 (the most recent estimate of AOE prevalence available), at an average cost of $200 per visit, including visit and prescription drug costs.3,12 Assuming that the prevalence of AOE has not changed and the proportion of systemic antimicrobial prescriptions for all AOE visits is similar to the proportion observed in this analysis, systemic antimicrobials could be prescribed for AOE more than 770,000 times each year (32.1% of 2.4 million). While the true percentage of visits for AOE involving complicating factors is unknown, increased use of topical preparations for uncomplicated AOE could prevent unnecessary use of systemic antimicrobials. Future work could explore the potential cost savings produced by using topical preparations alone for uncomplicated AOE.
Topical preparations are recommended for AOE in the absence of complicating factors: they are effective against P aeruginosa and S aureus and provide concentrated doses of antimicrobials effective even against resistant organisms.6 Penicillins (primarily amoxicillin) were prescribed in 42.3% of 2010 visits with a systemic antimicrobial prescription (a decline of 3.6% [95% CI −4.7, −2.4] from 2004). Amoxicillin is recommended for OM but is unlikely to be effective against P aeruginosa. OM and AOE can be difficult to distinguish, particularly when the tympanic membrane is not well visualized, which could explain the high percentage of penicillin prescriptions (53.8% of systemic antimicrobial prescriptions) by pediatricians despite the exclusion of visits with concurrent OM. However, the physiologic changes in the Eustachian tubes that occur with age suggest that the relative contribution of AOE to all ear infections could increase with age. More than half of AOE visits occurred in patients older than 14 years of,3,13 while the majority of acute OM has been estimated to occur in children younger than 5 years.14 Because pathogens and effective therapies differ for OM and AOE, increased consideration of AOE in adults and older children could be warranted.
One-quarter of 2010 ED visits resulted in a prescription for an opioid pain reliever. AOE can be exquisitely painful; pain assessment and management, including opioid use when necessary, is included in the guidelines.4 While ED clinicians had a higher proportion of prescriptions for opioids, it is likely that AOE patients visiting the ED were motivated to seek immediate treatment because of higher pain levels. However, because opioids have the potential for abuse and are an emerging cause of unintentional deaths,15 improved AOE prevention represents an opportunity for reducing opioid prescriptions.
This analysis is subject to multiple limitations. First, billing records, not medical records, were used. Systemic antimicrobial prescriptions could be appropriate, given other diagnoses not reflected in the billing record, and prescription drugs costing less than a copayment might be absent from the record. The analysis also relied on ICD-9 coding contained in the billing records. ICD-9 codes may be entered retrospectively by nonphysicians and may not fully reflect diagnoses recorded in the medical record.16,17 Second, the Marketscan database is a large convenience sample of insurers and contributors to the database changed over the study period. Because of this, no conclusions about trends in the overall prevalence of AOE or changes in the proportion of AOE visits treated by each specialty can be made. Third, although the guidelines call for pain assessment at each visit, this analysis was able to evaluate only prescriptions for pain relievers. We were unable to evaluate the actual rate of assessment of pain. In addition, we were unable to assess nonprescription treatments; thus, the prescription NSAID estimates are likely an underestimate of the true rate of NSAID use. Finally, this analysis included commercially insured patients. Treatment and prescribing patterns could differ for the uninsured or for patients with other insurance sources.
Improved implementation of clinical guidance could reduce the use of systemic antimicrobials when topical treatments are indicated. Use of systemic antimicrobials varied by specialty in this analysis, suggesting that guideline education efforts could be tailored to each clinician specialty. For example, AOE information was added to the American Academy of Pediatrics’ Red Book in 2012.18 Increased emphasis on patient education could also help prevent AOE. The Centers for Disease Control and Prevention has also made information and prevention materials for patients available.19–22 Greater adherence to the evidence-based clinical practice guidelines could reduce antimicrobial use, opioid use, and health care spending for a common, preventable disease.
Acknowledgments
The authors thank Lauri Hicks and Julia Gargano for helpful suggestions and thoughtful review of this manuscript.
Funding source: None.
Footnotes
Disclosures
Competing interests: None.
Sponsorships: None.
Author Contributions
Sarah A. Collier, acquisition of data, conception and design of study, analysis and interpretation of data, drafting the article, revising the article critically for important intellectual content, final approval; Michele C. Hlavsa, conception and design of study, analysis and interpretation of data, revising the article critically for important intellectual content, final approval; Emily W. Piercefield, conception and design of study, analysis and interpretation of data, revising the article critically for important intellectual content, final approval; Michael J. Beach, conception and design of study, analysis and interpretation of data, revising the article critically for important intellectual content, final approval.
References
- 1.Sander R. Otitis externa: a practical guide to treatment and prevention. Am Fam Physician. 2001;63:927–936. [PubMed] [Google Scholar]
- 2.Kaushik V, Malik T, Saeed SR. Interventions for acute otitis externa. Cochrane Database Syst Rev. 2010;1 doi: 10.1002/14651858.CD004740.pub2. CD004740. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Estimated burden of acute otitis externa—United States, 2003–2007. MMWR Morb Mortal Wkly Rep. 2011;60:605–609. [PubMed] [Google Scholar]
- 4.Rosenfeld RM, Brown L, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2006;134:S4–S23. doi: 10.1016/S0194-5998(06)00266-X. [DOI] [PubMed] [Google Scholar]
- 5.Hajioff D, Mackeith S. Otitis externa. Clin Evid (Online) 2010:510–532. [PMC free article] [PubMed] [Google Scholar]
- 6.Roland PS, Stroman DW. Microbiology of acute otitis externa. Laryngoscope. 2002;112:1166–1177. doi: 10.1097/00005537-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 7.McCoy SI, Zell ER, Besser RE. Antimicrobial prescribing for otitis externa in children. Pediatr Infect Dis J. 2004;23:181–183. doi: 10.1097/01.inf.0000109958.65053.4e. [DOI] [PubMed] [Google Scholar]
- 8.Halpern MT, Palmer CS, Seidlin M. Treatment patterns for otitis externa. J Am Board Fam Pract. 1999;1:1–7. doi: 10.3122/15572625-12-1-1. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya N, Kepnes LJ. Initial impact of the acute otitis externa clinical practice guideline on clinical care. Otolaryngol Head Neck Surg. 2011;145:414–417. doi: 10.1177/0194599811406797. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare and Research Quality. Prevention Quality Indicators Technical Specifications. Rockville, MD: AHRQ; 2011. [Google Scholar]
- 11.SAS Institute. Usage Note 37344: how can I estimate rate differences using a poisson model and get a confidence interval? [Accessed July 20, 2012];2009 Oct; http://support.sas.com/kb/37/344.html. [Google Scholar]
- 12.Collier SA, Stockman LJ, Hicks LA, et al. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol Infect. 2012;11:1–11. doi: 10.1017/S0950268811002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowlands S, Devalia H, Smith C, et al. Otitis externa in UK general practice: a survey using the UK General Practice Research Database. Br J Gen Pract. 2001;51:533–538. [PMC free article] [PubMed] [Google Scholar]
- 14.Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011:1487–1492. [PubMed] [Google Scholar]
- 16.Jones G, Taright N, Boelle PY, et al. Accuracy of ICD-10 codes for surveillance of Clostridium difficile infections, France. Emerg Infect Dis. 2012;18:979–981. doi: 10.3201/eid1806.111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell SE, Campbell MK, Grimshaw JM, et al. A systematic review of discharge coding accuracy. J Public Health Med. 2001;23:205–211. doi: 10.1093/pubmed/23.3.205. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics. Prevention of illnesses associated with recreational water use. In: Pickering LK, editor. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012. pp. 212–213. [Google Scholar]
- 19.Centers for Disease Control and Prevention. Swimmer’s ear: recreational water illness (RWI) [Accessed March 7, 2012]; http://www.cdc.gov/healthywater/swimming/rwi/illnesses/swimmers-ear.html.
- 20.Centers for Disease Control and Prevention. Facts about swimmer’s ear. [Accessed March 7, 2012]; http://www.cdc.gov/healthywater/pdf/swimming/resources/pseudomonas-factsheet_swimmers_ear.pdf.
- 21.Centers for Disease Control and Prevention. Swimmer’s ear prevention guidelines. [Accessed March 7, 2012]; http://www.cdc.gov/healthywater/swimming/rwi/illnesses/swimmers-ear-prevention-guidelines.html.
- 22.Centers for Disease Control and Prevention. Datos sobre la “foliculitis de la bañera” y el “oído de nadador.”. [Accessed March 7, 2012]; http://www.cdc.gov/healthywater/pdf/swimming/resources/pseudomonas-factsheet-esp.pdf.