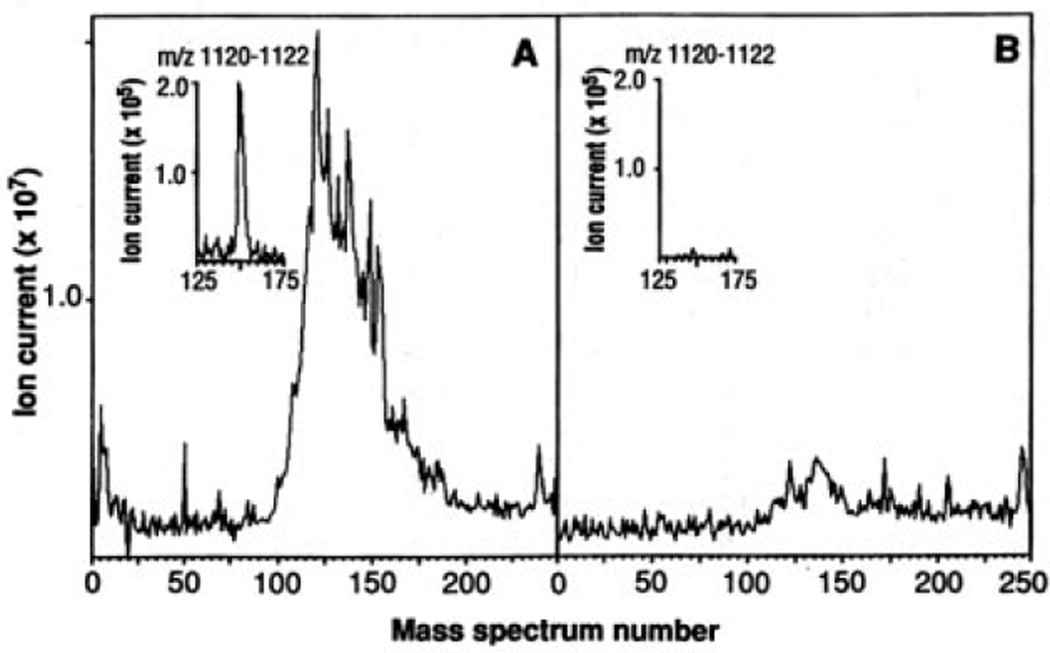
Fig. 1.
Total ion chromatograms of HLA-A2.1–associated peptides. C1R-A2.1 (A) or C1R (B) cells (2 × 109) were solubilized in 20 mM tris, pH 8.0, 150 mM NaCl, 1% NP-40, 2 mM PMSF, 100 µM iodoacetamide, Aprotinin (5 µg/ml), Leupeptin (10 µg/ml), Pepstatin A (10 µg/ml), EDTA (3 ng/ml), and 0.2% sodium azide. After sample centrifugation at 100,000g for 1 hour, supernatants were immunoprecipitated with the HLA-A2.1–specific monoclonal antibody BB7.2 (30) and protein A–Sepharose (Sigma). Precipitates were extracted with 0.2 N acetic acid. The supernatants were brought to pH 2.1 with glacial acetic acid and boiled for 5 min, centrifuged through an Ultrafree-Cl unit (millipore) with a norminal limit of 5 KD, and concentrated by vacuum centrifugation. Mass spectra were recorded on a Finnigan-MAT TSQ-70 (san Jose, California) triple quadrupole mass spectrometer equipped with an electrospray ion source and a C-18 microcapillary-HPLC column (75 µm by 10 cm) (14). The column was eluted with a gradient of 0 to 80% acetonitrile in 0.5% acetic acid over 10 min at 1 to 2 µl/min. The electrospray needle was operated with a voltage differential of 4 to 5keV and a sheath flow of 2 to 4 µl/min of 3:1 mixture of methanol:0.5% acetic acid. Total ion signal from samples equivalent to 108 cells was obtained by scanning the range of masses corresponding to m/z values between 300 and 1500 every 2 s and then summing each of the spectra. Insets are plots of ion current for the peptide (M+H)+ ions at m/z 1121 (nominal mass).