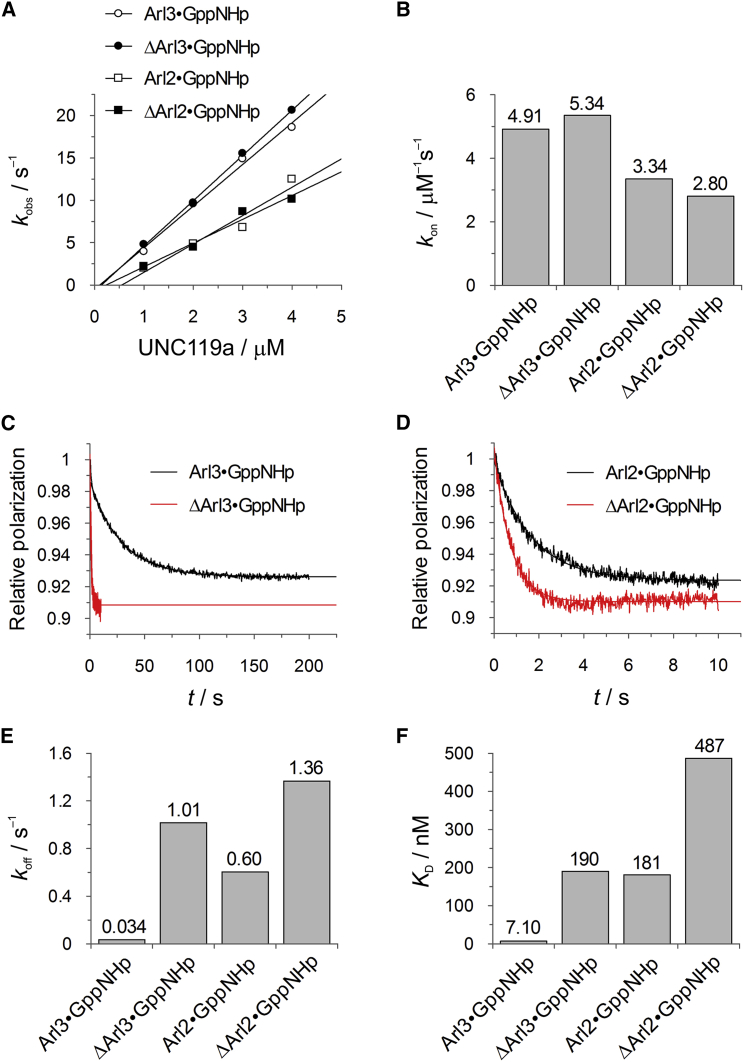
Figure 5.
Influence of the Arl3 N-terminal helix on UNC119a binding. (A) Stopped-flow fluorescence polarization kinetic measurements of the association of 0.2 μM mantGppNHp-loaded Arl proteins with increasing concentrations of UNC119a. The pseudo-first-order rate constants (kobs) thus obtained are plotted against the concentration of UNC119a. (B) Bar charts of the second-order association rate constants (kon) determined from the data given in (A). (C and D) In stopped-flow fluorescence polarization kinetic experiments, complexes of 2 μM UNC119a with 0.2 μM of mantGppNHp-loaded Arl proteins were mixed with a 200-fold excess of unlabeled Arl proteins to determine koff as indicated. (E) Bar charts of the dissociation rate constants (koff) determined in (C) and (D). (F) Equilibrium dissociation constants (KD) of Arl protein complexes with UNC119a as determined from the kinetic constants in (A)–(E) using koff/kon. To see this figure in color, go online.