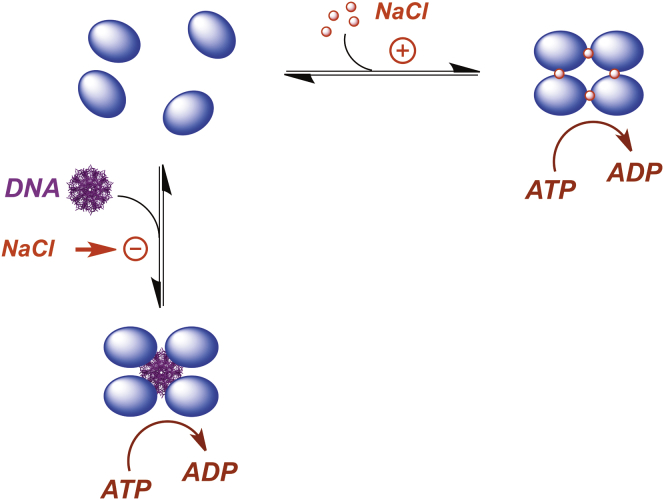
Figure 8.
Model for ion-linked (in vitro) and DNA-linked (in vivo) packaging motor assembly. (Blue oval) The terminase protomer is a stable complex composed of one large TerL subunit tightly associated with a dimer of TerS subunits, shown as a blue oval for simplicity. In vitro, assembly and activation of the ring tetramer motor is thermodynamically linked to salt binding. Under typical in vivo concentrations (100 nM), the protomer is the predominant species in solution. We suggest that the phosphate backbone of DNA mediates ring-tetramer assembly in vivo with concomitant activation of the packaging motor. Note that salt strongly inhibits terminase•DNA binding interactions. To see this figure in color, go online.