Figure 1. Complex DNA assembly using in vivo ligation by homologous recombination in yeast.
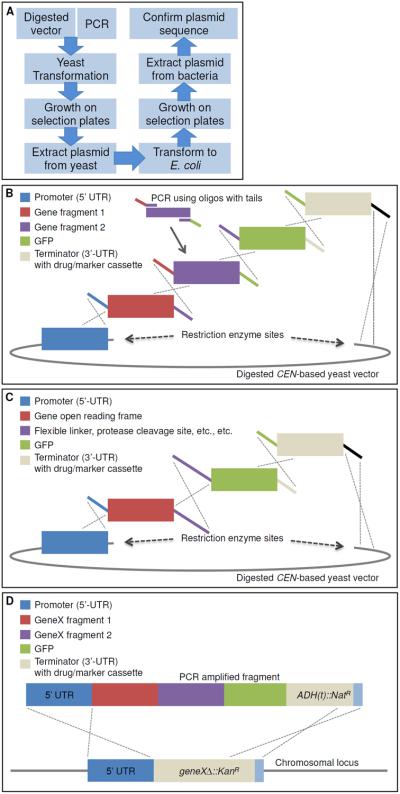
A. A diagram illustrating the workflow of the protocol to construct plasmids using homologous recombination in yeast. B. An example construct is illustrated using homologous overlapping primer tails (identical colors show homology and the dotted lines illustrate the resulting recombination event across the homologous segments). Digested CEN-based yeast vector, such as LEU2-based pRS315 (Sikorski and Hieter, 1989). This illustration represents a single example of a series of gene fragments assembled into a single construct in one step using in vivo ligation. Modifications to this arrangement can be used to assemble multiple domains within a single gene or genes, include multiple epitope or fluorescent tags at either the N- or C-termini, and/or include flanking UTR sequences for subsequent integration at a non-native chromosomal locus. C. This example specifically addresses a useful property of this process. Specifically, one can encode in the homologous overlapping tails between two adjacent gene fragments to be assembled any desired non-native sequence information to introduce additional sequences of interest (such as, but not limited to, an epitope tag, a Gly-Ser rich linker sequence, restriction enzyme cut sites, protein cleavage motifs, signal sequences, etc.). D. Following construction, the entire construct can be PCR-amplified and integrated into the genome in a single step using either designed homology (5'- and 3'-UTR) within the system or, in the case of the MX-4-based drug-resistance cassette system devised by Goldstein and McCusker (2001), homology between the common sequence (blue) installed downstream of each type of drug-resistance gene, in which ADH1(t) is the terminator sequence from the ADH1 locus. Replacement of the gene deletion cassette with the modified gene of interest also allows for a swap in the growth selection markers (either drug resistance or nutrient). This system can also be modified to accommodate essential genes, as long as the recipient cell also harbors a URA3-based vector expressing the WT essential gene from a second plasmid, which can be subsequently eliminated by counter-selection on 5-FOA medium (Boeke et al., 1984).
