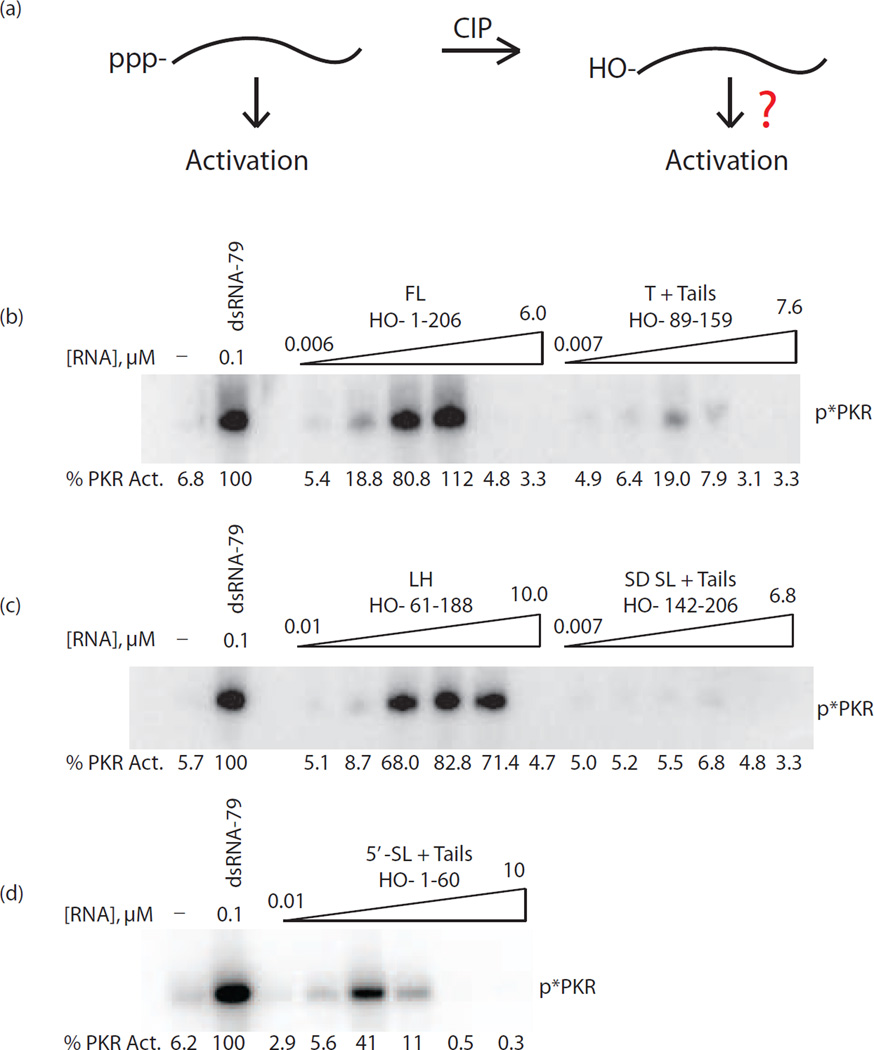
Figure 3.
Dependence of activation of PKR on 5’-triphosphate for full-length and truncated trp 5’-UTRs. (a) Model of activation by 5’-triphosphate RNA (left) and 5’-hydroxyl RNA (right). Calf-intestinal phosphatase (‘CIP’) is used to remove the 5’-triphosphate and leave a 5’-hydroxyl. In all RNA constructs tested, the 5’-triphosphate form activated PKR, but activation after CIP treatment was dependent on the size and structure of the RNA, as indicated by the question mark. (b–d) PKR activation assays of CIP-treated trp 5’-UTR constructs (b) FL (1–206) and T + tails, (89–159), (c) LH (61–188) and SD SL + tails (142–206), and (d) 5’-SL + tails (1–60). A buffer-only negative control is included and PKR activation is normalized to dsRNA-79 in each panel. Activation values are provided under each gel and the position of phosphorylated PKR is indicated as ‘p*PKR’. All assays were performed twice.