1. Introduction
Tubby mice carry a splicing mutation in the Tub gene which results in a malfunctioning intron coding protein (Bode and Wolfrum, 2003). Recently, a human ocular disease was shown to result from a mutation in the Tub gene and this disease exhibits the same phenotype as seen in tubby mice (Borman et al., 2014). One distinct characteristic of the tubby phenotype is the mislocalization of the photosensitive pigments (opsins) in the retina: they lose their polarized and restricted localization, and instead, the proteins are scattered throughout the photoreceptor cells (Cai et al., 2012; Kong et al., 2006; Sun et al., 2012). We have shown that oxidative stress is involved in, and accelerates, the retinal degeneration in tubby mice (Kong et al., 2006). Also prevention of photoreceptor cell death can be achieved via decreasing the amount of reactive oxygen species (ROS) by sulforaphane induced up regulation of antioxidant enzymes (Kong et al., 2007), overexpression of the thioredoxin (Trx) gene (Kong et al., 2010), or injection of catalytic nanoceria (Cai et al., 2012; Kong et al., 2011), which are inorganic direct antioxidants with properties mimicking superoxide dismutase and catalase (Karakoti et al., 2008).
ER (endoplasmic reticulum) stress has recently been implicated in many neurodegenerative diseases including ocular diseases and represents a distinct mechanism involved in cell death and other pathological activities (Cao and Kaufman, 2014; Sano and Reed, 2013; Tzekov et al., 2011). Over the past decade, targeting ER stress and the unfolded protein response (UPR) has emerged as a therapeutic strategy for a variety of disease treatments (Hetz et al., 2013; Kim et al., 2008). The ER is an important cellular organelle, which is responsible for protein synthesis, folding, secretion and Ca2+ homeostasis. The proper function of ER and its finely-tuned regulation of the maintenance of the proteome (refolding and/or degradation of the unfolded/misfolded proteins) play a critical role in determining cell survival or death (Cao and Kaufman, 2014; Szegezdi et al., 2006; Tzekov et al., 2011; Xu et al., 2005). Ample data demonstrate that many diseases are triggered by dysfunction of the protein quality control system in which accumulation of unfolded/misfolded proteins in the lumen of the ER, termed ER stress, causes the imbalance between the load of protein folding and ER capacity for protein folding (Chen et al., 2011). Oxidative stress has been reported to be the most dominant causative factor which cross-talks, interacts and is tightly linked with ER stress (Cao and Kaufman, 2014; Zhang, 2010). The UPR is initiated for self-protection in early ER stress conditions when the three ER stress sensors, PERK (protein kinase RNA-like ER kinase), ATF6 (activating transcription factor 6) and IRE1 (inositol-requiring enzyme 1), are activated following the release of GRP78/BiP (glucose regulated protein 78/binding immunoglobin protein). However, in cases of persistent ER stress conditions, increases in accumulation of malfolded proteins and failure of ER homeostasis re-establishment result in the impairment of the UPR. This eventually causes the UPR to switch its function from anti-apoptotic to pro-apoptotic and the apoptosis signaling pathway is initiated (Lai et al., 2007; Szegezdi et al., 2006) through regulation of caspases and CHOP (CCAAT/enhancer-binding protein homologous protein) expression.
It is logical to think that the mislocalization of photoreceptor-specific proteins in tubby mice might be indicative of the involvement of ER stress. To investigate the role of ER stress in the retinal degeneration in tubby mice, we examined the expression levels of ER stress markers, their dynamics, and the alteration of the components of the UPR during retinal development from P4 through P28. Furthermore, the expression of several apoptosis associated factors was analyzed to elucidate the mechanism of ER stress action for photoreceptor degeneration in tubby mice.
2. Materials and Methods
2.1 Animals
tubby mice on C57BL/6J background and wild type (wt, C57BL/6J) were purchased from Jackson laboratory and used as breeders for the colonies. Animals were maintained and genotyped as previously reported (Cai et al., 2012; Kong et al., 2006). Animal care and handling strictly follow the ARVO statement for the Use of Animals in Ophthalmic and Vision Research (ARVO), and the protocol for this study was approved by the Institutional Animal Care and Use Committee (IACUC) of University of Oklahoma Health Sciences Center and Dean A. McGee Eye Institute.
2.2 qRT-PCR and PCR array
Individual retinas (4 – 8) from tubby and C57BL/6J mice at postnatal day (P) P4 - P28 were collected; Total RNA isolation, cDNA reverse transcription, qRT-PCR performance and calculation of the relative expression of the target genes against the house-keeping gene GAPDH are the same as previously reported (Cai et al., 2012). Primers for mouse Grp78/BiP and Chop are the same as previously reported (Lin et al., 2007). PCR array was carried out using the “mouse unfolded protein response” array plates (SABiosciences). Changes in gene expression were shown as fold changes of tubby compared to wt (tub/wt) and fold changes between two groups of tubby (P28/P7) using the array software with P values indicated (SABiosciences).
2.3 Immunocytochemistry
The protocol we used for immunocytochemistry is the same as previously reported (Cai et al., 2012). Briefly, eyes were enucleated and fixed, the cornea and lens were removed. After cryo-protection, the eyecups were embedded and 10 μm thick sections were cut. The slides were blocked with 5% BSA, then incubated in the following primary antibodies for 2 hrs at room temperature: mouse anti-rhodopsin (R1D4) (1:2000, generous gift from Dr. Robert Molday, Columbia University, Vancouver, Canada), rabbit anti-M-opsin (1:500, Millipore, AB5405) and rabbit anti-GRP78 BiP (1:300, Abcam, ab21685). The slides were rinsed with PBS buffer and incubated with the secondary antibodies of goat anti-mouse AlexaFluor 594 or goat anti-rabbit AlexaFluor 488 for 1 hr at room temperature. After mounting with anti-fade Vectashield mounting media with DAPI, the slides were coverslipped, observed and imaged under a Nikon Eclipse 800 epi-fluorescence microscope using a 20x or a 40x lens with proper filters.
2.4 Western blot
Individual retinas (8–10) from P4 - P28 tubby and wt mice were collected, homogenized and proteins were quantified (Cai et al., 2012). Soluble proteins, 30 μg, were loaded in 10% SDS-page gels and electrophoresed. After electrophoretic transfer to the nitrocellulose membrane, the following primary antibodies at 1:1000 dilution were used: phospho-eIF2α (Ser51)(119A11) rabbit mAb and phospho-Bcl2 (Ser70)(5H2) rabbit mAb (Cell signaling technology, 3597 and 2827, respectively), mouse anti-ATF6 (Imgenex, IMG-273), rabbit anti-GRP78 BiP and rabbit anti-IRE1 (phospho S724) (Abcam, ab48187), rabbit NF-κB p65 (C-20) (Santa Cruz Biotechnology, sc-372) and mouse anti-XBP-1s (Biolegend, 647502). β-actin (13E5) rabbit mAb (HRP conjugate) (Cell signaling technology, 5125) was used as a loading control. The membrane development, imaging and densitometry of the bands were performed as previously reported (Cai et al., 2012; Kong et al., 2011).
2.5 Statistical analysis
Data are shown as mean ± SEM and indicated in each figure. The unpaired student t-test and/or one-way ANOVA with Bonferroni’s post hoc test for multiple comparison were performed and significant differences were determined by P<0.05.
3. Results
3.1 Mislocalization of rod and cone opsins in an early age
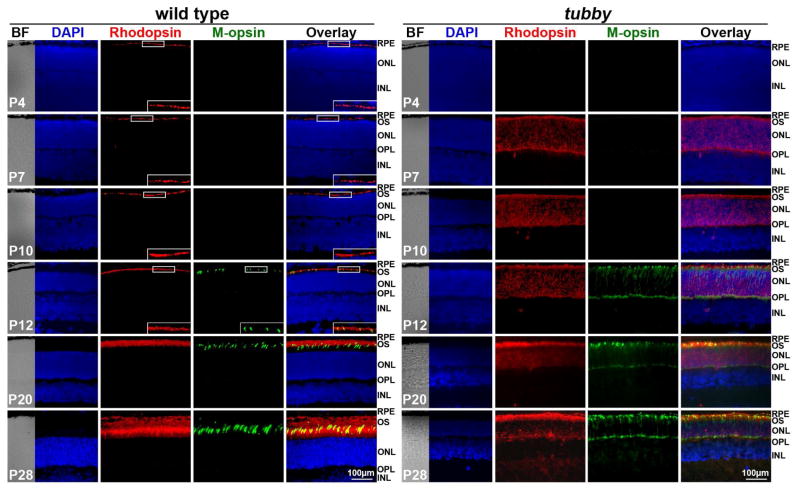
The rod and cone opsins, and other phototransduction-associated proteins have a polarized location in the membrane of retinal outer segment (OS) where they function in the visual cycle. We previously reported that rhodopsin, arrestin and α-transducin are mislocalized in tubby retinas when examined at P18 (Kong et al., 2006) and the same phenomenon was confirmed and extended to P28 or 1 month of age (Cai et al., 2012; Sun et al., 2012). However, there are no data indicating whether the protein mislocalization is a primary event or secondary response (they are transported to and distributed in the OS at the beginning of the OS formation but then the polarity is lost as the disease progresses). We performed immunocytochemistry to detect the expression and distribution of rod opsin (rhodopsin) and middle wave-length cone opsin (M-opsin) during retinal development (P4 - P28) in tubby and age-matched wt mice. Fig. 1 shows that rhodopsin in wt retinas was present at P4 as numerous small dots aligned in the nascent OS adjacent to the outer nuclear layer (ONL), while M-opsin was detectable at P12. The expression pattern of both proteins was retained and their levels increased with age. They reached the highest expression level and exhibited the typical expression pattern at P28. However in tubby retinas, rhodopsin appeared slightly later, compared to wt, at P7 and was widely distributed over the entire shorter OS, ONL and outer plexiform layer (OPL). M-opsin was also detectable at P12 in tubby retinas. In contrast to its proper localization (in the OS) in the wt, M-opsin in tubby retinas was localized in the short OS, ONL and OPL at P28. These data suggest that mislocalization of photoreceptor-specific proteins in tubby retinas is an early and possibly primary event following the loss of tubby function.
Figure 1.
Mislocalization of rhodopsin and M-opsin in tubby retinas. Immunocytochemistry of rhodopsin (red) and M-opsin (green) in wt (left) and tubby (right) retinas from P4 -P28 revealed that in wt retinas, rhodopsin is detectable at P4 and M-opsin at P12, whereas in tubby retinas, rhodopsin appears at P7 and M-opsin at P12 with mislocalization. Images shown are representative of 5–8 eyes per group. Nuclei were counterstained with DAPI. Inserts are the enlarged part of the boxed regions of the corresponding images. BF: bright field, RPE: retinal pigment epithelium, OS: outer segment, ONL: outer nuclear layer, OPL: outer plexiform layer, INL: inner nuclear layer. N=5–8, Scale bar, 100 μm.
3.2 Activities of ER stress markers during tubby retinal development
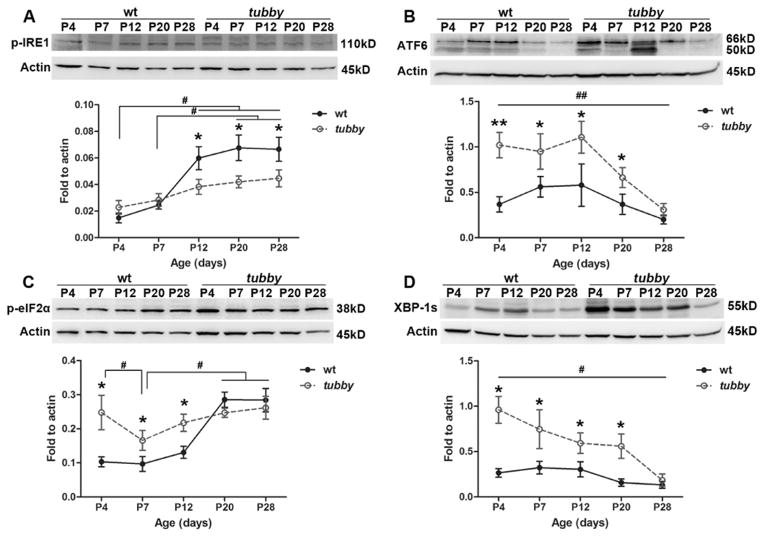
We have previously reported that oxidative stress is involved in tubby retinal degeneration (Kong et al., 2006). Logically, we think that ER stress is most likely also involved in this process because oxidative stress and ER stress have been reported to be tightly correlated and interactive with each other (Cao and Kaufman, 2014; Zhang, 2010). Based on our previous report that rapid photoreceptor loss occurs at P14 through P34 (Kong et al., 2006), western blots were performed to examine the expression of ER stress markers during retinal development (P4 - P28) using wt and tubby retinas. As shown in Fig. 2, the expression of activated IRE1 (p-IRE1) (Fig. 2A) in tubby retinas is slightly higher than age-matched wt at P4 and is lower than the wt at P12 and thereafter, although its expression level continues to be up regulated. Cleaved ATF6 level (Fig. 2B) in tubby retinas is up regulated compared to age-matched wt from P4 and its elevated level peaks at P12. Although ATF6 expression is decreased at P20, it is still greater than the wt. elF2α is downstream of PERK, so its activation (by phosphorylation) and level of expression directly reflect the level of phosphorylated PERK. Our data (Fig. 2C) demonstrated that p-eIF2α in tubby retinas is greater than wt at P4 through P12 and becomes less than wt at P20 through P28.
Figure 2.
Dynamic changes of ER stress markers during retinal development. Western blots show the expression of p-IRE1 (A), ATF6 (B), p-eIF2α (C), and XBP-1s (D) at selected developmental ages for wt and tubby retinas. Representative bands from each group are shown. Densitometric analysis of the bands is standardized to actin and shown as mean ± SEM beneath each western blot figure. N=8–10, *P<0.05, **P<0.005 (compared between tubby and age-matched wt using the unpaired student t-test), #P<0.05, ##P<0.005 (compared among tubby at different ages using one way ANOVA for B and D, or compared between each two groups using the unpaired student t-test for A and C).
XBP-1 (transcription factor X-box binding protein) is the downstream transcription factor of phosphorylated IRE1. Its active form (spliced XBP-1, XBP-1s) binds to the promoters of its targeted genes to regulate their expression and help in refolding and degrading the unfolded/misfolded proteins (Lee et al., 2003). In the current study, western blot assay (Fig. 2D) shows that XBP-1s is up regulated at P4 through P20 in tubby retinas compared to age-matched wt, although its expression gradually decreased to the wt level at P28.
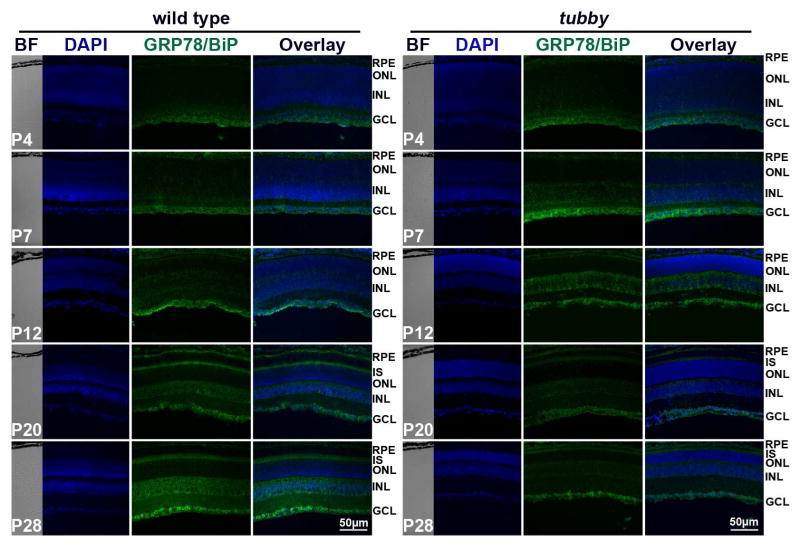
GRP78/BiP is an ER resident chaperone protein and a key regulator of the UPR. Its cytoprotective/anti-apoptotic role has been reported in many tissues in a variety of diseases (Gorbatyuk et al., 2010; Gorbatyuk et al., 2012; Morris et al., 1997; Pfaffenbach and Lee, 2011). To determine the dynamics of GRP78/BiP during retinal development, western blots and qRT-PCR were performed using P4 - P28 retinas from wt and tubby. Fig. 3 shows that the expression of GRP78/BiP protein and mRNA levels in tubby retinas are greater than the age-matched wt at P4 and peaks at P12. Its expression is lower than the wt and younger tubby mice at P20 through P28 (Fig. 3A–C). The expression and distribution of GRP78/BiP in the retinas were also assessed by immunocytochemistry (Fig. 4). The results show that weak expression of GRP78/BiP was seen in the RPE and it was stronger in the ganglion cell layer (GCL) in P4 wt retinas. It further distributed into the INL at P7. GRP78/BiP was distinct and strongly expressed in the RPE, IS, INL and GCL at P20 through P28. The expression pattern of GRP78/BiP in tubby retinas was similar to the wt. However, the fluorescence intensity in tubby retinas was more than the wt at P4 through P12, and the fluorescence intensity was greatly reduced in tubby retinas at P20 and thereafter (Fig. 4).
Figure 3.
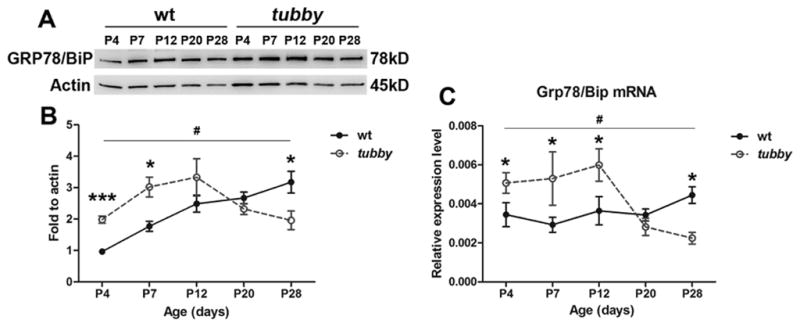
Dynamics of GRP78/BiP expression during retinal development. GRP78/BiP protein is increased at P4 through P12 in tubby retinas compared to age-matched wt, but has significantly decreased expression at P20 through P28. (A) Data shown are representative of 8–10 western blots and (B) the average (mean ± SEM) of band intensity from 8–10 retinas. (C) qRT-PCR analysis demonstrated a similar alteration of Grp78/Bip mRNA level (mean ± SEM). *P<0.05, ***P<0.0001 (compared between tubby and age-matched wt using the unpaired student t-test), #P<0.05 (compared among tubby at different ages using one way ANOVA).
Figure 4.
Distribution and alteration of GRP78/BiP protein during retinal development. Immunocytochemistry showed that in wt and tubby retinas, GRP78/BiP is mainly expressed in the RPE, INL and GCL at P4 through P12 with expression being stronger in tubby retinas than the wt, but at P20 through P28, it was also expressed in the IS. However, tubby retinas have much less GRP78/BiP fluorescence compared to wt and to younger tubby retinas at P20 and P28. Representative images from each group are shown. BF: bright field, RPE: retinal pigment epithelium, IS: inner segment, ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer. N=8–10, Scale bar, 50 μm.
3.3 UPR behavior during tubby retinal development
Given the results from western blot and immunocytochemistry assessment, we performed PCR array to assay the alterations of mRNA levels of the UPR components during retinal degeneration by selecting P7 and P28 retinas and using the “mouse unfolded protein response” array plates. A total of 89 genes were surveyed for the expression of ER stress-associated signaling. The genes to be tested on the array plates can be categorized as the following groups: 1) unfolded protein binding and/or protein folding (Canx, Cct4, Dnajb2, Dnajb9, Ero1l, Ero1lb, Hspa4l, Htra2, Scap, Sec63, Tcp1, Tor1a, Uggt1); 2) ER protein folding quality control (Edem1, Edem3, Ganab, Rpn1, Serp1, Uggt1); 3) protein disulfide isomerization (PDI) (Ddit3, Ero1l, Ero1lb, H47, Pdia3, Srebf1); 4) ER associated degradation (ERAD) (Amfr, Derl1, Derl2, Edem1, Fbxo6, H47, Herpud1, Mbtps1, Mbtps2, Nploc4, Nucb1, Os9, Sec62, Syvn1, Ubxn4); 5) heat shock proteins (Dnajb2, Dnajb9, Dnajc3, Hspa1l, Hsph1, Sec63); 6) ubiquitination (Amfr, Edem3, Herpud1, Rnf139, Rnf5, Sec62, Ube2g2, Ube2j2, Ubxn4, Ufd1l); 7) regulation of translation (Eif2α, Insig1, pp1r15b, Serp1); 8) transcription factors (Atf4, Atf6, Atxn3, Cebpb, Creb3, Creb3I3, Ddit3, Ern2, Mbtps1, Scap, Srebf1, Xbp1); and 9) apoptosis (Bax, Atxn3, Cebpb, Ddit3, Ern2, H47, Htra2, Manf, Mapk8, Mapk10, Pdia3, Ppp1r15b). In P7 tubby retinas, 44% of the surveyed genes (37 out of 84 genes) changed their expression (only those >1.5 fold are listed). Over 20% of them (Atf4, H47, Hspa1l, Mbtps1, Rnf5, Sec62, Sec63, Ube2j2) increased their expression by more than 2 fold compared to age-matched wt (Table 1). The remaining genes (28 of 37) had large changes (>1.5 but <2 fold) (Table 2). However, at P28, the majority of these UPR genes in tubby retinas were greatly down regulated compared to P7 tubby retinas. Among these genes, transcription factors and regulators of translation, such as Atf4, Atf6 and Eif2α, were up regulated at P7 in tubby retinas by 2.83, 1.88 and 1.32 fold compared to age-matched wt (tub/wt). However, they were down regulated in P28 tubby retinas by 2.54, 1.79 and 2.28 fold compared to P7 tubby retinas (tubP28/P7).
Table 1.
Mouse unfolded protein response genes with changes in expression in tubby mice (≥ 2.0 fold).
| Symbol | Gene name | Function | tub/wt(P7) | tub(P28/P7) | ||
|---|---|---|---|---|---|---|
| fold | P value | fold | P value | |||
| Atf4 | Activating transcription factor 4 | g | 2.83 | 0.1301 | −2.54 | 0.0140* |
| Bax | Bcl2-associated X protein | k | 1.15 | 0.4472 | −2.95 | 0.0012** |
| Canx | Calnexin | a,b | 1.63 | 0.3210 | −2.06 | 0.2201 |
| Cct4 | Chaperonin containing Tcp1, subunit 4 (delta) | a,b | 1.90 | 0.1393 | −2.49 | 0.0139* |
| Derl1 | Der1-like domain family, member 1 | e | 1.49 | 0.3704 | −2.01 | 0.0740 |
| Eif2a | Eukaryotic translation initiation factor 2a | f | 1.32 | 0.3732 | −2.28 | 0.0179* |
| Ern2 | Endoplasmic reticulum (ER) to nucleus signalling 2 | g,k | 1.60 | 0.1774 | −3.24 | 0.0479* |
| Ganab | Alpha glucosidase 2 alpha neutral subunit | c | 1.34 | 0.0180* | −2.13 | 0.0253* |
| H47 | Histocompatibility 47 | e, h, k | 2.30 | 0.0671 | −1.33 | 0.1094 |
| Hspa1l | Heat shock protein 1-like | j | 1.96 | 0.1665 | −2.12 | 0.2263 |
| Mbtps1 | Membrane-bound transcription factor peptidase, site 1 | e, g, i | 2.47 | 0.0019** | −2.19 | 0.0002** |
| Rnf5 | Ring finger protein 5 | d | 2.12 | 0.1821 | −1.55 | 0.1063 |
| Sec62 | SEC62 homolog (S. cerevisiae) | e, d | 2.62 | 0.0734 | −1.73 | 0.0399* |
| Sec63 | SEC63-like (S. cerevisiae) | a, b, j | 3.06 | 0.0203* | −1.65 | 0.0328* |
| Serp1 | Stress-associated endoplasmic reticulum protein 1 | c, f | 1.43 | 0.0530 | −2.27 | 0.0016** |
| Ube2j2 | Ubiquitin-conjugating enzyme E2, J2 homolog (yeast) | d | 2.90 | 0.0085** | −2.19 | 0.0290* |
| Creb3l3 | CAMP responsive element binding protein 3-like 3 | g | 1.76 | 0.2692 | 1.97 | 0.0335* |
| Dnajb2 | DnaJ (Hsp40) homolog, subfamily B, member 2 | a, b, j | 1.36 | 0.4107 | 2.37 | 0.0008** |
| Insig1 | Insulin induced gene 1 | f | 1.65 | 0.3395 | 3.92 | 0.0125* |
| Nploc4 | Nuclear protein localization 4 homolog (S. cerevisiae) | e | 1.37 | 0.3617 | 1.95 | 0.0864 |
| Cebpb | CCAAT/enhancer binding protein (C/EBP), beta | g,k | −1.13 | 0.5732 | 2.69 | 0.0381* |
| Dnajb9 | DnaJ (Hsp40) homolog, subfamily B, member 9 | a, b, j | −1.70 | 0.0978 | 2.52 | 0.0108* |
| Edem3 | ER degradation enhancer, mannosidase alpha-like 3 | c, d | −2.14 | 0.2194 | 2.77 | 0.0211* |
| Syvn1 | Synovial apoptosis inhibitor 1, synoviolin | e | −1.21 | 0.3540 | 2.01 | 0.0440* |
N=3–4,
P<0.05,
P<0.01
Unfolding protein binding;
Protein folding;
ER protein folding quality control;
Ubiquitination;
ER associated degradation (ERAD);
Regulation of translation;
Transcription factor;
Protein disulfide isomerization (PDI);
Regulation of cholesterol metabolism;
Heat shock protein;
Apoptosis
Table 2.
Mouse unfolded protein response genes with changes in expression in tubby mice (≥ 1.5 fold< 2.0 fold).
| Symbol | Gene name | Function | tub/wt(P7) | tub(P28/P7) | ||
|---|---|---|---|---|---|---|
| fold | P value | fold | P value | |||
| Amfr | Autocrine motility factor receptor | e,d | 1.64 | 0.0888 | −1.87 | 0.0708 |
| Manf | Mesencephalic astrocyte-derived neurotrophic factor | k | −1.31 | 0.4786 | −1.81 | 0.2844 |
| Atf6 | Activating transcription factor 6 | g | 1.88 | 0.2989 | −1.79 | 0.3563 |
| Atxn3 | Ataxin 3 | g,k | 1.59 | 0.2715 | −1.35 | 0.3849 |
| Creb3 | CAMP responsive element binding protein 3 | g | 1.85 | 0.1243 | −1.83 | 0.1152 |
| Derl2 | Der1-like domain family, member 2 | e | 1.51 | 0.0379* | −1.49 | 0.0171* |
| Dnajc3 | DnaJ (Hsp40) homolog, subfamily C, member 3 | j | 1.84 | 0.0401* | −1.52 | 0.0471* |
| Edem1 | ER degradation enhancer, mannosidase alpha-like 1 | c,e | 1.64 | 0.0360* | −1.34 | 0.0249* |
| Fbxo6 | F-box protein 6 | e | 1.75 | 0.0040** | 1.15 | 0.5788 |
| Htra2 | HtrA serine peptidase 2 | a,e, k | 1.40 | 0.3803 | −1.41 | 0.0012* |
| Mapk8 | Mitogen-activated protein kinase 8 | k | 1.21 | 0.6105 | −1.70 | 0.0423* |
| Mbtps2 | Membrane-bound transcription factor peptidase, site 2 | i, e | 1.64 | 0.1199 | −1.65 | 0.1137 |
| Nucb1 | Nucleobindin 1 | e | 1.70 | 0.3499 | −1.80 | 0.3027 |
| Rnf139 | Ring finger protein 139 | d | 1.05 | 0.9995 | −1.67 | 0.0090** |
| Rpn1 | Ribophorin I | c | 1.50 | 0.1036 | −1.27 | 0.0540* |
| Scap | SREBF chaperone | a, g | 1.86 | 0.1465 | −1.42 | 0.0108* |
| Srebf1 | Sterol regulatory element binding transcription factor 1 | g, h, i | 1.91 | 0.0569 | −1.72 | 0.0876 |
| Tcp1 | T-complex protein 1 | a, b | 1.14 | 0.1114 | −1.67 | 0.0039* |
| Tor1a | Torsin family 1, member A (torsin A) | a, b | 1.21 | 0.4038 | −1.57 | 0.0316* |
| Ube2g2 | Ubiquitin-conjugating enzyme E2G 2 | d | −1.82 | 0.1978 | −1.18 | 0.1275 |
| Ubxn4 | UBX domain protein 4 | e, d | 1.83 | 0.0915 | −1.34 | 0.1228 |
| Ufd1l | Ubiquitin fusion degradation 1 like | d | 1.28 | 0.3043 | −1.93 | 0.0278* |
| Uggt1 | UDP-glucose glycoprotein glucosyltransferase 1 | a, b, c | 1.57 | 0.0876 | −1.54 | 0.0419* |
| Ddit3 | DNA-damage inducible transcript 3 | g, h, k | 1.48 | 0.0528 | 1.61 | 0.0492* |
| Herpud1 | Homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 | d, e | 1.91 | 0.0893 | 1.74 | 0.0066** |
| Hspa4l | Heat shock protein 4 like | b | 1.35 | 0.1475 | 1.80 | 0.0234* |
| Hsph1 | Heat shock 105kDa/110kDa protein 1 | j | 1.71 | 0.3653 | 1.39 | 0.3202 |
| Mapk10 | Mitogen-activated protein kinase 10 | k | 1.43 | 0.1494 | 1.81 | 0.0153* |
| Os9 | Amplified in osteosarcoma | e | 1.74 | 0.2344 | 1.83 | 0.0622 |
| Pdia3 | Protein disulfide isomerase associated 3 | h, k | 1.71 | 0.2060 | 1.28 | 0.3002 |
| Ero1l | ERO1-like (S. cerevisiae) | b, h | −1.42 | 0.2597 | 1.57 | 0.0278* |
| Ero1lb | ERO1-like beta (S. cerevisiae) | a, h | −1.17 | 0.5778 | 1.66 | 0.0340* |
| Ppp1r15b | Protein phosphatase 1, regulatory (inhibitor) subunit 15b | f, k | −1.30 | 0.4214 | 1.51 | 0.0582 |
| Xbp1 | X-box binding protein 1 | g | −1.46 | 0.5196 | 1.47 | 0.0983 |
N=3–4,
P<0.05,
P<0.01
Unfolding protein binding;
Protein folding;
ER protein folding quality control;
Ubiquitination;
ER associated degradation (ERAD);
Regulation of translation;
Transcription factor;
Protein disulfide isomerization (PDI);
Regulation of cholesterol metabolism;
Heat shock protein;
Apoptosis
3.4 Expression of apoptotic regulators/effectors
It is well accepted that photoreceptor death results from the impairment of the balance between anti-apoptotic and pro-apoptotic effectors. To determine the mechanism(s) by which the photoreceptor death is regulated, the expression of apoptotic regulators/effectors, such as B-cell lymphoma protein 2 (Bcl2), CHOP and nuclear factor kappa B (NF-κB), was examined by qRT-PCR or western blots using age-matched wt and tubby retinas at P4 - P28. As shown in Fig. 5, phosphorylated Bcl2 (Fig. 5A) in tubby retinas was significantly down regulated compared to the wt at P7 and remained there. Chop (Fig. 5C) expression in tubby retinas was greater than that of wt litter mates at P7 through P28. NF-κB is a key effector for promoting apoptosis (Khandelwal et al., 2011) and has been reported to be activated by ER stress and ROS (Kaneko et al., 2003; Pahl and Baeuerle, 1997). In the current study, our data show that NF-κB (Fig. 5B) expression is up regulated in tubby compared to age-matched wt at P4 and P7, but its level is similar to wt at P12 through P28.
Figure 5.
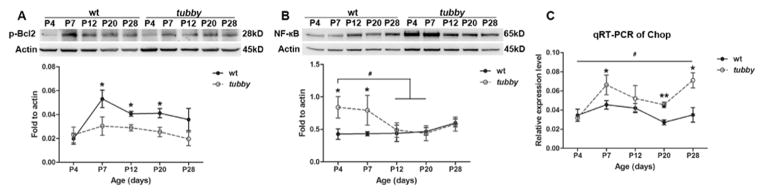
Expression of anti-apoptotic and pro-apoptotic effectors/regulators during retinal development. Western blot assay demonstrated that the activation of p-Bcl2 (A) decreases with age. The expression of NF-κB (B) is increased in the early developmental stages in tubby retinas (P4 and P7), but decreases thereafter. Densitometric analysis of the bands is shown beneath the corresponding western figure. (C) qRT-PCR analysis showed that the level of Chop mRNA in tubby is higher than the wt from P4 through P28. Data shown are mean ± SEM, N=8–10. *P<0.05, **P<0.005 (compared between tubby and age-matched wt using the unpaired student t-test), #P<0.05 (compared among tubby at different ages using one way ANOVA for A and C, or compared between each two groups using the unpaired student t-test for B).
4. Discussion
The mislocalization of the key phototransduction proteins (rhodopsin, M-opsin and arrestin) is a distinct characteristic of tubby phenotype (Cai et al., 2012; Kong et al., 2006; Sun et al., 2012). In this study, we showed that the mislocalization of rhodopsin and M-opsin in tubby retinas occurs as soon as they are synthesized at P4 and P12 respectively and the expression of the majority of the UPR components is increased at P7. This suggests that mutation of the Tub gene results in disturbance of the normal distribution of rod and cone opsins and that this event triggers the activation of the UPR signaling cascade. The expression of ER stress markers, IRE1, eIF2α and GRP78/BiP is elevated which demonstrated that ER stress is indeed involved in the process of tubby retinal development. Mislocalization or aggregation of photoreceptor-specific proteins caused by the mutations in ocular genes has been shown to be a very common phenomenon, for example, in Rpe65−/− (Rohrer et al., 2005), Lrat−/− (Zhang et al., 2011), Cng3A−/− and Cng3B−/− (Thapa et al., 2012), Rp2null (Li et al., 2013) and Gnat2c.518A>G (Jobling et al., 2013) mice. Increases in ER stress and activation of the UPR signaling were also reported in Lart−/− (Zhang et al., 2011) and Cng3−/− (Thapa et al., 2012) mice.
Three ER-resident transmembrane proteins (PERK, ATF6 and IRE1) are the sensors responding to ER stress. By activation (phosphorylation or cleavage), these sensor proteins initiate a self-defense UPR signaling network to regulate their downstream gene transcriptional and translational activities for maintaining the homeostasis and proper function of the ER (Doyle et al., 2011; Lai et al., 2007; Salminen et al., 2010). A recent publication reported that, by selective chemical-genetic activation of individual PERK, ATF6 or IRE1 pathways in HEK293 cell lines expressing Fv2E-PERK, TetON-ATF6f or IRE1[1642G], respectively, the aggregation of mutant rhodopsin was reduced (Chiang et al., 2012). Surprisingly, PERK also reduced the level of wt rhodopsin (Chiang et al., 2012). Many eye diseases including retinitis pigmentosa (Kunte et al., 2012; Shinde et al., 2012; Yang et al., 2007), age-related macular degeneration (AMD) (Salminen et al., 2010), glaucoma (Zode et al., 2014), oxygen-induced retinopathy (OIR) (Liu et al., 2013) and diabetic retinopathy (DR) (Yan et al., 2012), etc., have been shown to involve ER stress in their pathology. In the current study, the expression of ER stress markers and their dynamics during retinal development in tubby mice were assessed using wt as a reference. Our data demonstrated that IRE1, ATF6, eIF2α, XBP-1s and GRP78/BiP were up regulated in tubby retinas at early developmental stages (P4 through P12 or P20) compared to age-matched wt, and they were down regulated thereafter. Similar observations were also obtained from transgenic P23H (at P6) (Lin et al., 2007), hT17M Rho (at P15) (Kunte et al., 2012) and rd1 mice (at P10) (Yang et al., 2007), and in the ER stress-associated indicator (ERAI)-transgenic mice expressing a variant of the gene for green fluorescent protein (F-XBP1DDBD-venus), in which fluorescent intensity increased after treatment with an ER stress inducer (Shimazawa et al., 2007). However, persistent ER stress caused decreased expression of these markers (Lin et al., 2007). The regulation of the UPR genes assayed with the “mouse unfolded protein response” PCR arrays (Table 1 and 2) was also in agreement with this result. In P7 tubby retinas (before the peak of photoreceptor death), a majority of the UPR genes, especially those having functions related to unfolding protein binding and protein folding, ER protein folding quality control, ER associated degradation (ERAD) and protein disulfide isomerization (PDI), showed increased expression levels. However, P28 retinas (an age after the peak of photoreceptor death) exhibited down regulation of these genes (Table 1 and 2). It should be noted that the heat shock protein family 70 (HSP70) is an important group of proteins for normal cellular activities. One group of the HSP70 family, CCT (Chaperonin containing Tcp1), was shown to be essential for elongation and extension of the rod outer segments, i.e. mice deficient in CCT were unable to build outer segments due to the impairment of ciliary trafficking (Posokhova et al., 2011; Sinha et al., 2014). It has been reported that tubby mice exhibited blockage of transport of selective G protein-coupled receptors (GPCRs) to the neuronal primary cilia and aberrant rhodopsin trafficking, indicating that the Tubby protein is a required part of the transport machinery in retinal cells (Sun et al., 2012). These discoveries, in combination with our PCR array data, suggest that the primary cause of retinal degeneration in tubby retinas is the mislocalization of key phototransduction proteins which induces ER stress. The activated UPR signaling is an attempt to overcome the earlier ER stress condition in tubby retinas. However, the unmitigated prolonged ER stress triggers dysregulation of UPR genes, which in turn promotes the failure of trafficking and transportation of necessary components (structural proteins) for photoreceptor build up. Moreover, our data indicate that all three pathways of the UPR are elevated in the tubby retinas and ER stress involvement in tubby retinal degeneration is in a time window similar to that of apoptosis, in which TUNEL-positive apoptotic photoreceptor cells increased from P14 through P19 and decreased by P28 (Bode and Wolfrum, 2003), and also agrees with our report that significant loss of photoreceptors occurs in tubby at P14 through P34 (Kong et al., 2006).
The important role of the IRE1/XBP-1 pathway in mediating cell death and inflammatory signaling is well established (Tang et al., 2014). XBP-1 activation was reported to have a protective role in preventing injury-induced retinal ganglion cell death (Hu et al., 2012). Overexpression of spliced XBP-1 (XBP-1s) stimulated cell proliferation and inhibited ER stress-induced CHOP-involving apoptosis, whereas its knockdown reversed these effects (Guo et al., 2012). Overexpression of ATF6 up regulated IRE1 spliced XBP-1, which was negatively regulating apoptosis by down regulation of caspases, JNK and CHOP (Guo et al., 2014). In our current study, IRE1 expression was slightly higher in the tubby mutant retinas at P4 compared to wt; it continued to increase but was lower than age-matched wt at P12 and thereafter. ATF6 and XBP-1s are up regulated in comparison to age-matched wt at P4 through P20, although it tended to decrease with age and return to the wt level at P28. This suggests their adaptive protective role occurs at an early developmental age with the loss of function at a later developmental stage following a prolonged ER stress condition in tubby retinas.
Although how ER stress induces apoptosis is not well defined, potential mechanisms involving multiple signaling pathways have been proposed and shown to involve the proteases (caspase 12), kinases (ASK1, JNK, and P38MAPK), transcription factors (CHOP) and Bcl2 family members (Lin et al., 2007; Nakanishi et al., 2013; Xu et al., 2005). It is commonly accepted that the communication between the ER and mitochondria for apoptosis is predominantly mediated by the Bcl2 family of proteins (Doyle et al., 2011). The anti-apoptotic Bcl2 protein protects against oxidative stress-induced damage and maintains intracellular Ca2+ homeostasis, which in turn controls the release of Ca2+ from the ER to the cytoplasm as a secondary messenger to prevent apoptosis (Rong and Distelhorst, 2008; Szegezdi et al., 2009). CHOP can be induced by all three UPR pathways. It can be regulated by ATF6 and XBP-1 through their binding to the promoter region of the Chop gene (Yoshida et al., 2001), and CHOP mediated cell death is directly linked with repressing the expression of Bcl2 protein (McCullough et al., 2001). Moreover, knockdown of Chop mRNA inhibits light-induced photoreceptor cell death (Nakanishi et al., 2013). NF-κB was activated by an ER overload (Pahl and Baeuerle, 1997) and it is the common downstream effector activated by both IRE1 and eIF2α (Hung et al., 2004; Jiang et al., 2003; Kaneko et al., 2003). In the current study, we showed that p-Bcl2 was down regulated and CHOP and NF-κB were up regulated in tubby retinas. These data suggest that retinal degeneration in tubby mice results from the imbalance of the expression of multiple anti-apoptotic and pro-apoptotic regulator/effectors. In particular, persistent down regulation of Bcl2 protein and up regulation of CHOP and NF-κB expression contribute to the apoptotic death of photoreceptor cells, although transient increases in the expression of protective GRP78/BiP, ATF6 and XBP-1s occurred during earlier retinal degeneration.
It should be emphasized that GRP78/BiP expression was up regulated in tubby mice compared to age-matched wt at P4 until P12, and was down regulated thereafter. Accumulated data suggest that GRP78/BiP is a neuroprotector and survival factor which prevents neurodegeneration (Gorbatyuk et al., 2010; Gorbatyuk et al., 2012; Morris et al., 1997; Pfaffenbach and Lee, 2011). As a new strategy for a therapeutic reagent, GRP78/BiP has been shown to successfully rescue retinal structure and function in P23H rats (Gorbatyuk et al., 2010), and other forms of neurodegenerative diseases (Inokuchi et al., 2009). We think that supplementation of the GRP78/BiP protein before its expression is decreased, or a combination of increased GRP78/BiP protein with other antioxidants, will inhibit retinal degeneration in tubby mice.
5. Conclusion
Our data demonstrate that ER stress is involved in tubby retinal degeneration and the dynamics of ER stress markers match the time window of photoreceptor death in tubby mice. This finding supports potential therapeutic strategies for treatment of tubby retinal degeneration by up regulation or down regulation of specific ER stress markers before significant changes occur during tubby retinal development.
Protein mislocalization in tubby retinal development correlates with ER stress
Dynamics of ER stress parallel temporal degeneration of tubby retina
Potential mechanisms for retinal degeneration are proposed
Acknowledgments
The authors thank the personnel at the animal, imaging and molecular modules of the Vision Research Core Facility at the Oklahoma University Health Sciences Center. The authors also thank Jiali Dong for his technical assistance.
Financial Support: This work was supported in part by NIH NEI P30 EY021725, R21EY018306, R01EY018724 and R01EY022111, National Science Foundation: CBET-0708172, COBRE-P20 RR017703-10 (XC). The funding resources had no role in study design, data collection, analysis and interpretation, manuscript preparation and decision to submit the manuscript for publication.
Footnotes
Disclosure statement: There is no potential conflict of interest for all the authors listed in this paper.
Author contributions: Conceived and designed the experiments: XC, JFM. Performed the experiments and collected the data: XC, LC. Analyzed and interpreted the data: XC, LC, JFM. Wrote the paper: XC, JFM.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bode C, Wolfrum U. Caspase-3 inhibitor reduces apototic photoreceptor cell death during inherited retinal degeneration in tubby mice. Mol Vis. 2003;9:144–50. [PubMed] [Google Scholar]
- Borman AD, et al. A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Hum Mutat. 2014;35:289–93. doi: 10.1002/humu.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, et al. Sustained protection against photoreceptor degeneration in tubby mice by intravitreal injection of nanoceria. Biomaterials. 2012;33:8771–81. doi: 10.1016/j.biomaterials.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, et al. Cellular strategies of protein quality control. Cold Spring Harb Perspect Biol. 2011;3:a004374. doi: 10.1101/cshperspect.a004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WC, et al. Selective activation of ATF6 and PERK endoplasmic reticulum stress signaling pathways prevent mutant rhodopsin accumulation. Invest Ophthalmol Vis Sci. 2012;53:7159–66. doi: 10.1167/iovs.12-10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KM, et al. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med. 2011;15:2025–39. doi: 10.1111/j.1582-4934.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk MS, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci U S A. 2010;107:5961–6. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk MS, et al. Glucose regulated protein 78 diminishes alpha-synuclein neurotoxicity in a rat model of Parkinson disease. Mol Ther. 2012;20:1327–37. doi: 10.1038/mt.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FJ, et al. ATF6 upregulates XBP1S and inhibits ER stress-mediated apoptosis in osteoarthritis cartilage. Cell Signal. 2014;26:332–42. doi: 10.1016/j.cellsig.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Guo FJ, et al. XBP1S protects cells from ER stress-induced apoptosis through Erk1/2 signaling pathway involving CHOP. Histochem Cell Biol. 2012;138:447–60. doi: 10.1007/s00418-012-0967-7. [DOI] [PubMed] [Google Scholar]
- Hetz C, et al. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12:703–19. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- Hu Y, et al. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73:445–52. doi: 10.1016/j.neuron.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung JH, et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004;279:46384–92. doi: 10.1074/jbc.M403568200. [DOI] [PubMed] [Google Scholar]
- Inokuchi Y, et al. Effect of an inducer of BiP, a molecular chaperone, on endoplasmic reticulum (ER) stress-induced retinal cell death. Invest Ophthalmol Vis Sci. 2009;50:334–44. doi: 10.1167/iovs.08-2123. [DOI] [PubMed] [Google Scholar]
- Jiang HY, et al. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23:5651–63. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling AI, et al. A naturally occurring mouse model of achromatopsia: characterization of the mutation in cone transducin and subsequent retinal phenotype. Invest Ophthalmol Vis Sci. 2013;54:3350–9. doi: 10.1167/iovs.13-11831. [DOI] [PubMed] [Google Scholar]
- Kaneko M, et al. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931–5. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- Karakoti AS, et al. Nanoceria as Antioxidant: Synthesis and Biomedical Applications. JOM (1989) 2008;60:33–37. doi: 10.1007/s11837-008-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal N, et al. Nucleolar NF-kappaB/RelA mediates apoptosis by causing cytoplasmic relocalization of nucleophosmin. Cell Death Differ. 2011;18:1889–903. doi: 10.1038/cdd.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, et al. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Kong L, et al. Neuroprotective effect of overexpression of thioredoxin on photoreceptor degeneration in Tubby mice. Neurobiol Dis. 2010;38:446–55. doi: 10.1016/j.nbd.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, et al. Nanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathways. Neurobiol Dis. 2011;42:514–23. doi: 10.1016/j.nbd.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, et al. Bright cyclic light accelerates photoreceptor cell degeneration in tubby mice. Neurobiol Dis. 2006;21:468–77. doi: 10.1016/j.nbd.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Kong L, et al. Delay of photoreceptor degeneration in tubby mouse by sulforaphane. J Neurochem. 2007;101:1041–52. doi: 10.1111/j.1471-4159.2007.04481.x. [DOI] [PubMed] [Google Scholar]
- Kunte MM, et al. ER stress is involved in T17M rhodopsin-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53:3792–800. doi: 10.1167/iovs.11-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E, et al. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- Lee AH, et al. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. Ablation of the X-linked retinitis pigmentosa 2 (Rp2) gene in mice results in opsin mislocalization and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2013;54:4503–11. doi: 10.1167/iovs.13-12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, et al. Targeting the IRE1alpha/XBP1 and ATF6 arms of the unfolded protein response enhances VEGF blockade to prevent retinal and choroidal neovascularization. Am J Pathol. 2013;182:1412–24. doi: 10.1016/j.ajpath.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KD, et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, et al. Immunoglobulin binding protein (BiP) function is required to protect cells from endoplasmic reticulum stress but is not required for the secretion of selective proteins. J Biol Chem. 1997;272:4327–34. doi: 10.1074/jbc.272.7.4327. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, et al. Role of endoplasmic reticulum stress in light-induced photoreceptor degeneration in mice. J Neurochem. 2013;125:111–24. doi: 10.1111/jnc.12116. [DOI] [PubMed] [Google Scholar]
- Pahl HL, Baeuerle PA. The ER-overload response: activation of NF-kappa B. Trends Biochem Sci. 1997;22:63–7. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–6. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posokhova E, et al. Disruption of the chaperonin containing TCP-1 function affects protein networks essential for rod outer segment morphogenesis and survival. Mol Cell Proteomics. 2011;10:M110 000570. doi: 10.1074/mcp.M110.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, et al. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46:3876–82. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- Salminen A, et al. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010;16:535–42. doi: 10.2119/molmed.2010.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–70. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazawa M, et al. Involvement of ER stress in retinal cell death. Mol Vis. 2007;13:578–87. [PMC free article] [PubMed] [Google Scholar]
- Shinde VM, et al. ER stress in retinal degeneration in S334ter Rho rats. PLoS One. 2012;7:e33266. doi: 10.1371/journal.pone.0033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, et al. Essential role of the chaperonin CCT in rod outer segment biogenesis. Invest Ophthalmol Vis Sci. 2014;55:3775–85. doi: 10.1167/iovs.14-13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, et al. Tubby is required for trafficking G protein-coupled receptors to neuronal cilia. Cilia. 2012;1:21. doi: 10.1186/2046-2530-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, et al. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, et al. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941–53. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- Tang CH, et al. Inhibition of ER stress-associated IRE-1/XBP-1 pathway reduces leukemic cell survival. J Clin Invest. 2014;124:2585–98. doi: 10.1172/JCI73448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa A, et al. Endoplasmic reticulum stress-associated cone photoreceptor degeneration in cyclic nucleotide-gated channel deficiency. J Biol Chem. 2012;287:18018–29. doi: 10.1074/jbc.M112.342220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzekov R, et al. Protein misfolding and retinal degeneration. Cold Spring Harb Perspect Biol. 2011;3:a007492. doi: 10.1101/cshperspect.a007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, et al. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, et al. Expression of endoplasmic reticulum stress-related factors in the retinas of diabetic rats. Exp Diabetes Res. 2012;2012:743780. doi: 10.1155/2012/743780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LP, et al. Activation of endoplasmic reticulum stress in degenerating photoreceptors of the rd1 mouse. Invest Ophthalmol Vis Sci. 2007;48:5191–8. doi: 10.1167/iovs.07-0512. [DOI] [PubMed] [Google Scholar]
- Yoshida H, et al. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3:33–40. [PMC free article] [PubMed] [Google Scholar]
- Zhang T, et al. Cone opsin determines the time course of cone photoreceptor degeneration in Leber congenital amaurosis. Proc Natl Acad Sci U S A. 2011;108:8879–84. doi: 10.1073/pnas.1017127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zode GS, et al. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest. 2014;124:1956–65. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]