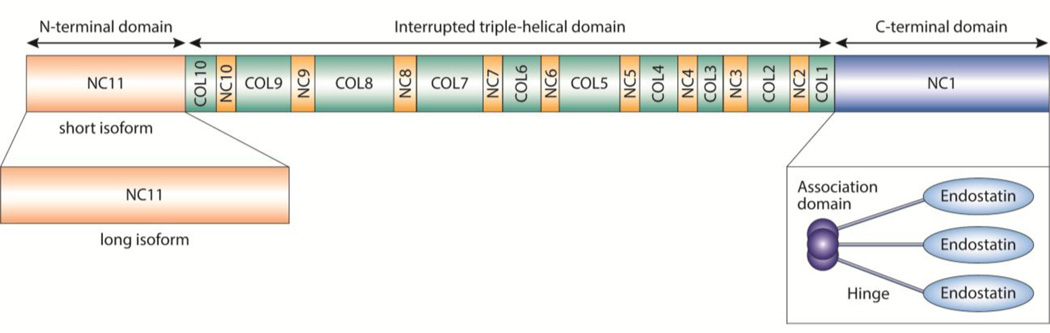
FIGURE 1.
Schematic model of the α1 chain of human collagen XVIII. The human collagen XV α1 chain is structurally homologous. They belong to a collagen subfamily, the multiplexins, on the basis of their central triple-helical domain (green boxes) interrupted by non-collagenous sequences. They contain an extended non-collagenous N-terminal domain, which, in collagen XVIII, can undergo alternative splicing (pale orange boxes), and a non-collagenous C-terminal domain (NC1; blue). The homotrimers (dark blue circles); a hinge domain (blue lines), which is highly susceptible to proteolytic processing; and a C-terminal endostatin domain (blue ovals), which has angiostatic properties. [Adapted from Iozzo [37] with permission from Nature Publishing Group.]