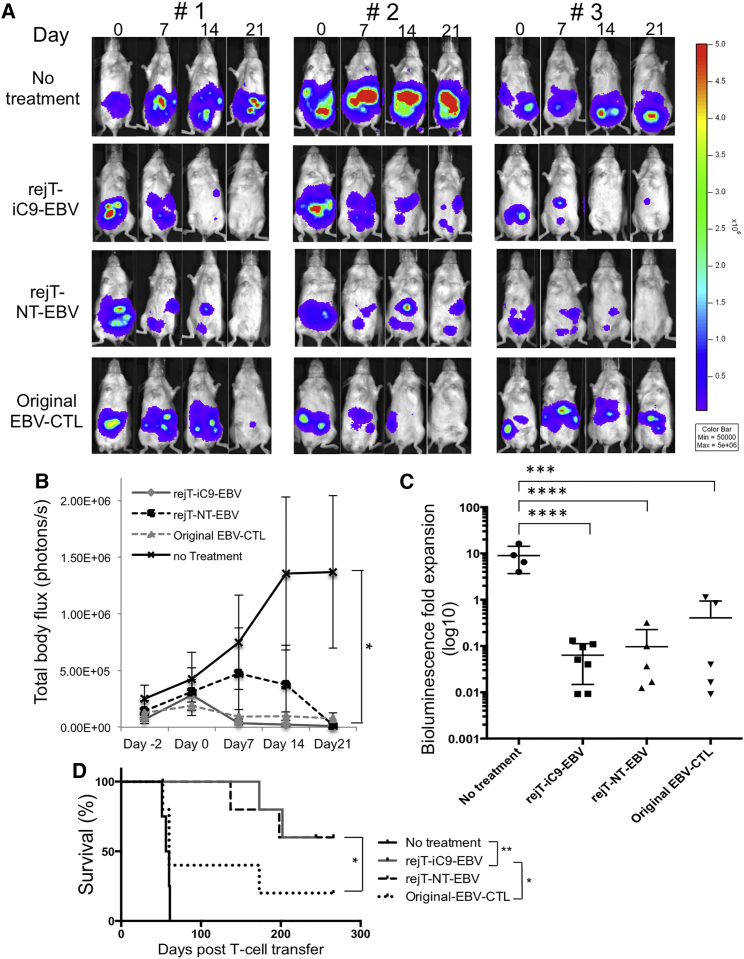
Figure 4.
In Vivo Antitumor Effect of iC9-iPSC-Derived EBV-CTLs
(A) NOD-Scid mice were inoculated intraperitoneally with HLA-A∗02-positive EBV-LCL cells labeled with GFP/FFluc and then treated either with control or EBV-CTL lines. Mice were divided into three groups that around 5 days later received rejT-iC9-EBV (n = 7), rejT-NT-EBV (n = 5), or original EBV-CTLs (n = 5). “No treatment” indicates that mice were injected with EBV-LCLs but not with CTLs (n = 4). Images of three representative mice from each group are shown.
(B) Total body flux (photons/s) for each mouse was quantified, and group averages were calculated. Error bars represent ± SEM. ∗p < 0.05 by one-way ANOVA comparing no treatment to rejT-iC9-EBV, rejT-NT-EBV, or original EBV-CTLs.
(C) Total tumor growth by day 21 after CTL infusions is represented as log10 signal change. Error bars represent ± SD. ∗∗∗∗p < 0.0001 and ∗∗∗p < 0.001 by one-way ANOVA.
(D) Kaplan-Meier survival curves for treated and control mice (rejT-iC9-EBV, n = 7; rejT-NT-EBV, n = 5; original EBV-CTLs, n = 5; no treatment, n = 4). ∗∗p < 0.01 and ∗p < 0.05 by the Gehan-Breslow-Wilcoxon test.