Figure 1.
An Overview of the Manufacturing of Human Induced Pluripotent Stem Cells under cGMPs
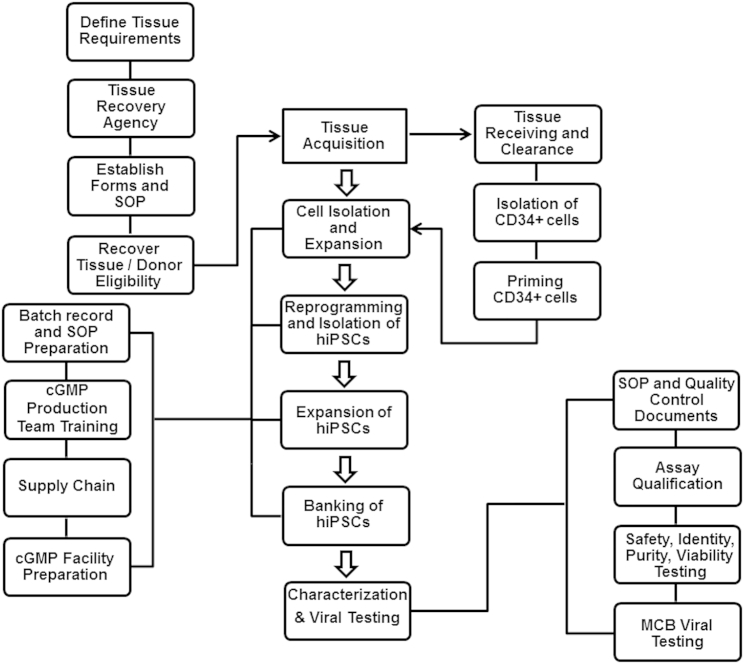
A tissue acquisition program was established, focusing on defining tissue requirements, working with a tissue recovery agency, establishing forms and standard operating procedures, recovering tissue, and donor eligibility determination. The manufacturing started with the isolation of CD34+ cells from a fresh cord blood unit and continued to priming, expansion, and then reprogramming of CD34+ cells. After generation of iPSCs and expansion, the cells were banked and eventually tested. Every step of the manufacturing process was documented and performed according to the batch records and standard procedures. Following characterization of the final bank, the results were reviewed by the quality assurance group to release the GMP iPSC lot.