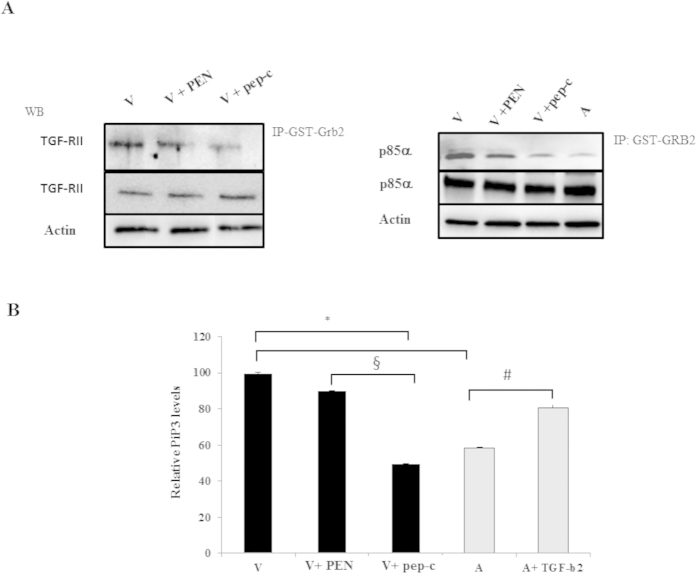
Figure 2. Grb2 associates with TGF-RII and the p85 subunit of PI3-K.
(A) Left panel. Pull-down assay performed with TGF-RII receptor expressed by Theileria-transformed macrophages incubated with GST-Grb2 beads. Bottom two rows demonstrate equivalent amounts of actin and TGF-RII in the cell extracts mixed with the GST-Grb2 beads. The top row demonstrates more TGF-RII is bound to GST-Grb2 in virulent macrophages and binding is not diminished by PEN treatment. TGF-RII binding is ablated when Grb2 SH3-interactions are dampened by treatment with peptidimer-c. Right panel. Immunoprecipitation analysis with anti-p85 antibody using whole cell lysates derived from virulent macrophages (V) incubated with GST-Grb2 beads overnight. Bottom two rows indicate that equivalent amounts of cell extract containing p85α and actin were mixed with GST-Grb2 beads. Top row shows the amount of p85α bound to GST-Grb2 beads in virulent and attenuated macrophages. Treatment of virulent macrophages with PEN did not diminish the amount of p85α bound to GST-Grb2. Ablation of SH3 domain interactions of Grb2 with peptidimer-c in virulent macrophages diminishes Grb2 binding of p85α to attenuated levels. (B) Ablating Grb2 partner-interactions with peptidimer-c reduces PI3-K activity to attenuated levels. PI3-K activity in virulent macrophages is decreased when virulent macrophages are treated with 1 μM of peptidimer-c, and adding exogenous TGF-β2 to attenuated macrophages increases PI3-K activity. *p < 0.05 compared to virulent macrophages, #p < 0.05 compared to attenuated macrophages, §p < 0.05 compared to virulent macrophages treated with PEN.