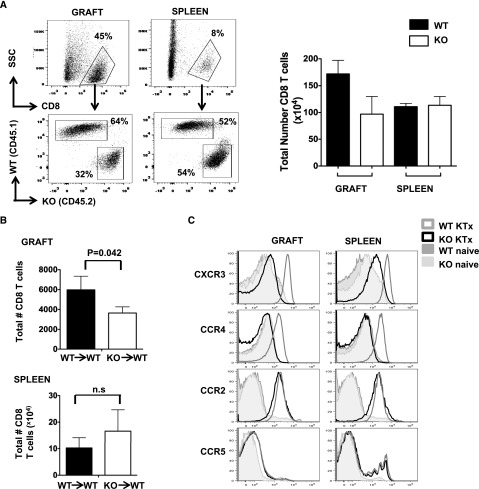
Figure 6.
CD8 T cells from MyD88−/− mice are defective in homing to the kidney allograft. (A) RAG−/− mice on a BALB/c background (CD45.2+) received kidney allografts from B6 donor mice. The mice were allowed to recover for 14 days before CFSE-labeled naive BALB/c WT (CD45.1+) and MyD88−/− (CD45.2+) CD8 T cells were cotransferred at a 1:1 ratio (5×106 WT and 5×106 MyD88−/− cells/mouse intravenously). Ten days after adoptive transfer, the kidney grafts and spleens were harvested and the percentage (representative dot plots on left) and absolute number (bar graph on right) of congenically marked WT and MyD88−/− CD8 T cells were determined by flow cytometry. Results are representative of two to three independent experiments. (B) CFSE labeled congenic CD8 T cells derived from naive WT (CD45.1) or naive MyD88−/− (CD45.2) mice were adoptively transferred into the indicated WT or MyD88−/− immunocompetent BALB/c recipient mice (5×106/mouse intravenously). Twenty-four hours after adoptive transfer, the recipient mice received B6 kidney allografts. Ten to 12 days after transplant, the kidney grafts and spleens were harvested and the absolute number of congenically marked donor CD8 T cells were determined. Results are representative of four independent experiments. (C) Adoptively transferred CD8 T cells present in the spleens or kidney allografts from RAG−/− recipient mice as in part A were analyzed for chemokine expression. For each histogram, cells were first gated on total CD8 T cells followed by gating of the specific congenic markers CD45.1+ (WT) or CD45.2+ (MyD88−/−). Naive CD8 T cells before adoptive transfer are shown for comparison (open dark gray histogram, WT; shaded histogram, MyD88−/−). Results are representative of three independent experiments. KO, knockout; KTx, kidney transplant.