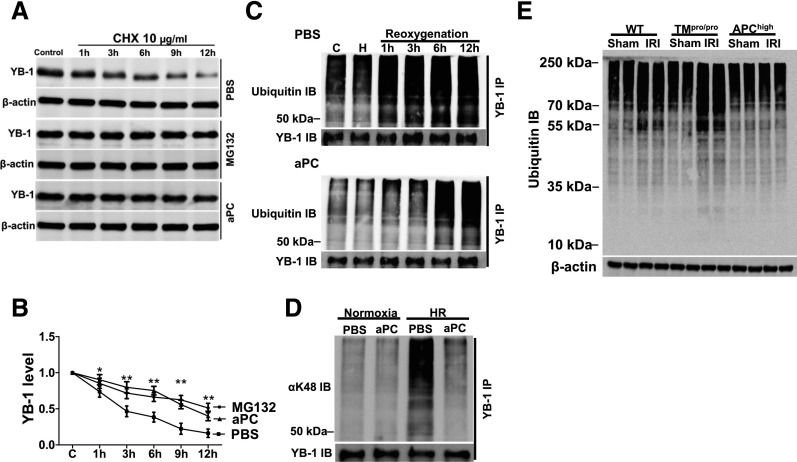
Figure 4.
aPC suppresses YB-1 ubiquitination. (A) and (B) aPC increases the protein stability of YB-1. After treatment of BUMPT cells with the protein synthesis inhibitor CHX (10 µg/ml) YB-1 protein levels markedly decline. Treatment of BUMPT cells with the proteasome inhibitor MG132 (10 µM) or aPC (20 nM) stabilizes YB-1 levels when compared with PBS-treated control cells; representative immunoblots (IBs) of YB-1 in whole-cell lysates (A) and line graphs (B) summarizing results. (C) YB-1 is rapidly ubiquitinated after in vitro reoxygenation (top, PBS-treated control). Pretreatment with aPC (20 nM, bottom) delays and reduces YB-1 ubiquitination. Immunoprecipitation (IP) of YB-1 from whole-cell lysates and representative images of ubiquitin IBs and YB-1 IB as input control. (D) HR (6 hours postreoxygenation) induces YB-1 K48–linked ubiquitination in BUMPT cells, which is efficiently suppressed by aPC treatment (20 nM). IP of YB-1 from whole-cell lysates and representative images of IBs using an antibody against K48-linked ubiquitin (top, K48-linked ubiquitin IB; bottom, YB-1 IB as input control). (E) After IRI, protein ubiquitination is increased in WT mice, and to a larger extent in TMPro/Pro, but not in APChigh mice. Representative IB of ubiquitinated proteins (top) in kidney lysates; β-actin as loading control (bottom). Mean±SD value of at least three independent experiments (B); *P<0.05; **P<0.01 (ANOVA).