Abstract
Diverticular disease (DD) is one of the most prevalent gastrointestinal disorders. The pathogenesis of diverticulosis and DD is controversially discussed. Current studies call the traditional concept of a fibre-deficient diet causing the development of diverticula into question. Data from two recent twin studies have provided conclusive evidence for a strong genetic component to diverticulosis. Although genomewide association studies have provided new insights into the polygenic architecture of human diseases, genomic research in diverticulosis and DD has just been started. This is an astonishing fact given the high morbidity and mortality of the disease, as well as the substantial economic burden on health care systems. For this review, we provide an update of the molecular pathobiology and summarise recent evidence supporting the hypothesis that distinct, yet unidentified genetic variants contribute to the development of diverticulosis and DD.
Keywords: Diverticular disease, diverticulosis, genetics, genomewide association studies, personalised medicine, polygenic disease, polymorphism, risk prediction
Introduction
Colonic diverticula are acquired submucosal herniations through weak areas of the intestinal smooth muscle such as blood vessel entry sites. Diverticular disease (DD) represents a serious burden to health care systems. Recent cost estimates in the United States (US) were reported to be $2.7 billion.1 Estimates of DD-associated mortality in Europe indicated that 23,000 deaths occur per year,2 and the prevalence has been rising further. Though mostly asymptomatic, a subgroup of patients develop associated morbidities, in particular irritable bowel syndrome (IBS)-like symptoms (symptomatic DD), which is also referred to as post-diverticulitis-IBS.3 The term diverticulitis is used to describe inflammation of the diverticula. It is generally estimated that at least 10% of patients with diverticulosis develop diverticulitis during their lifetime, but a recent large retrospective study from the US reported a lower incidence of about 4%.4 Diverticulitis can lead to potentially life-threatening complications such as colonic perforation, abscess formation, or bowel obstruction.
Aside from aging, previous studies have identified different environmental factors as being risk factors for the formation of colonic diverticula in the gut. Recent epidemiological data point to genetic factors contributing to the development and progression of DD. To date, the inherent genetic risk remains unknown. Therefore the aim of this article is to review recent studies to summarise the current evidence on the pathophysiology of diverticulosis and DD with a particular focus on genetics, as two recent landmark twin studies5,6 provide conclusive evidence for genetic risk factors. Currently, there is no widely accepted definition of the different terms of the condition, and they are used inconsistently by different authors. Box 1 summarises the terminology used throughout the review to define diverticulosis, DD and its complications.
Box 1.
Glossary
| Diverticulosis |
| Structural abnormality of the bowel with submucosal herniations through weak areas of the smooth muscle without clinical symptoms |
| Diverticular disease (DD) |
| Diverticulosis, in which symptoms and/or complications develop; this subsumes symptomatic diverticular disease (irritable-bowel-syndrome (IBS)-like symptoms such as postdiverticulitis-IBS), diverticulitis and complicated DD |
| Symptomatic diverticular disease |
| Persistent or recurrent symptoms without signs of diverticulitis or other complications |
| Diverticulitis |
| Inflammation of a diverticulum causing abdominal pain accompanied by raised inflammation markers |
| Complicated diverticular disease |
| Formation of an abscess or fistula, colonic perforation, stenosis or bleeding due to inflamed diverticula |
Environmental factors
Fibre
The hypothesis that DD is a diet-related disease of Western civilisation was introduced by Painter and Burkitt in the 1960s based on their observation that diverticulosis was common in urbanised countries and rare in rural Africa.7 The difference was attributed to the fact that a low-fibre diet reduces stool volume, decreases colon diameter, increases intraluminal pressure on the colonic wall in accordance with Laplace's law, and thus creates diverticula. This was followed by several observational studies evaluating the role of dietary fibre.8–10 The evaluation of the influential role of dietary constituents in health and disease is complex, and these studies are limited by selection bias, the inherent difficulties in accurately capturing dietary intake, possible inadequate accounting for confounding variables, and different diagnostic modalities for the assessment of the prevalence of diverticula. Two large cross-sectional studies by Peery et al. could not find a protective effect of dietary fibre: In their cross-sectional study containing 2108 patients from the Vitamin D and Calcium Polyp Prevention study, a fibre-rich diet was not associated with a decreased risk for diverticulosis11 (odds ratio (OR) 0.96); in their second cross-sectional study containing 2104 patients from the Diet and Health Study those patients within the highest quartile of fibre intake were found to have greatest prevalence of diverticulosis.12 In conclusion, the traditional protective role of fibre in the pathogenesis of diverticulosis and DD is currently being called into question. The differentiating role of the fibre subtypes (soluble/insoluble) has not been adequately investigated; nevertheless, some data suggest that insoluble fibre might be more protective.12 With respect to (symptomatic) DD, the Health Professionals Follow-up Study (HPFS)13 comprising 51,000 US male health professionals and the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC)14 consisting of 57,000 patients from the United Kingdom (UK) found a protective effect of a fibre-rich diet. This finding is reinforced by data from the prospective Million Women Study from the UK, which observed a reduced incidence of DD on a high-fibre diet; this finding did, however, depend on the specific sources of fibre, with the lowest risk resulting from fruit consumption.15
Non-fibre dietary constituents
Additionally, several non-fibre dietary constituents have been linked to diverticulosis and DD:
Vitamin D
Vitamin D regulates intestinal proliferation and is important to maintain colonic homeostasis by modulating inflammation. Among patients with diverticulosis, higher levels of 25-hydroxyvitamin D are associated with a lower risk of diverticulitis.16 This is supported by findings from a large study showing low ultraviolet (UV) light exposure is associated with diverticulitis.17 The molecular mechanisms behind the role of vitamin D in (complicated) DD have to date not been investigated but could be related to its anti-inflammatory effects.18
Vegetarian diet
The vegetarian diet causes an altered colonic microbiota and has been linked to protection from colon cancer. In the EPIC cohort, after adjusting for important confounding variables such as dietary intake of fibre, a reduced risk for DD was found in vegetarians.13 Similar observations were made in a previous study by Gear et al.19
Red meat
Red meat is another dietary risk factor for colon cancer and obesity and was assessed in several studies. In the HPFS and EPIC cohorts, increased intake of red meat conferred a higher risk for DD13 and symptomatic DD.14 Although this is supported by smaller studies,8,20 a recent cross-sectional analysis by Peery et al.12 found no association between the intake of red meat and the prevalence of diverticulosis.
Nuts and corn
Mechanistic considerations link indigestible nutrient constituents with DD. It is hypothesised that they might occlude diverticula and therefore cause DD and particularly diverticulitis. A large prospective study in the HPFS cohort found inverse associations between nut and popcorn consumption and the risk of diverticulitis: Nuts and corn consumption all reduced the risk for diverticulitis and diverticular bleeding.21 This, however, still needs to be confirmed by further studies.
Smoking
Smoking is a proinflammatory stimulus and the major preventable and modifiable cause of death worldwide. A prospective study found an increased incidence of symptomatic DD in women who were smokers.22 Moreover, a higher frequency of complicated DD may be present in smokers, as evidenced in several large case series.23,24
Physical activity
Increased physical activity decreases colonic transit time and colonic pressure and thus is speculated to reduce the incidence of diverticulosis and DD. In the Diet and Health Study with 2104 participants, Peery et al.12 found no association between physical activity and diverticulosis. In contrast, physical activity is reported to lower the risk of complications of DD including diverticulitis and diverticular bleeding.25,26
Obesity
The frequency of DD has increased steadily concomitant with elevated rates of obesity during the past decades. Obesity is a well-known risk factor for many digestive disorders such as colon cancer, gallstone disease, and gastro-oesophageal reflux. An increased body mass index (BMI) has been linked to an elevated risk for diverticulitis and complicated DD in large prospective series. Rosemar et al.,27 Strate et al.28 and Hjern et al.26 reported large prospective population-based series that show an association between complications of DD and obesity. The role of obesity in the development of diverticulosis was investigated in three studies: Song et al.29 and Strate et al.28 could not find an association, in contrast to the Israeli study by Kopylov et al.30 (3175 patients), which found an association between obesity and diverticulosis using multivariate analysis.
Constipation
Constipation is speculated to be associated with the development of diverticula by increased pressure on the colonic wall, yet recent studies have found no evidence for a diverticulogenic role of constipation;11,12 therefore, constipation should not be considered a risk factor at present.
Drugs
The utilisation of opiate analgesics, corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs) has been associated with complications of DD in several studies.31–33 The strongest data are available for NSAIDs, as evidenced in a recent meta-analysis34 summarising 11 studies for diverticular perforation (OR 1.46–10.5 with a pooled increased odds of 3.4) and 12 studies for diverticular bleeding (OR 1.0–15.6 with a pooled increased odds of 2.7). The largest study within the HPFS cohort found an increased risk for diverticulitis and diverticular bleeding with regular use of aspirin or NSAIDs.35
Genetic factors
Epidemiologic studies: at-risk populations
The actual prevalence of colonic diverticulosis is difficult to determine because most individuals with colonic diverticula are asymptomatic.4 Epidemiological studies report remarkable variations in prevalence rates and predominant location of diverticula depending on ethnicity. Additionally, several inherited diseases of the connective tissue have been connected with DD and diverticulosis: Ehlers-Danlos syndrome (EDS) type IV, Williams-Beuren syndrome, polycystic kidney disease, Coffin-Lowry syndrome, and possibly Marfan syndrome.
Ethnicity
Despite the fact that the prevalence of diverticula is difficult to assess and only limited data exist, it is generally accepted that diverticula in Western countries are predominantly localised in the left colon, whereas in Asian countries they occur predominantly in the right colon.36 Studies in multiracial populations sharing similar living conditions have also found ethnic differences in prevalence rates.37–39
From the prevalence rates of diverticulosis and DD in different ethnicities, data on ethnic subgroups can be aggregated. Table 1 summarises available information on the location and frequency of diverticulosis in different ethnicities.
Table 1.
Selected controlled cross-sectional surveys with more than 200 participants
| Population | Yeara | Predominant location | Number of patients | Prevalence | Mean age (years) | Reference | Gender ratio (M:F) | Diagnostic modality | Additional information |
|---|---|---|---|---|---|---|---|---|---|
| Africa | |||||||||
| Kenya | 1978 | N/A | 226 | 6.6% | 40 | Calder40 | N/A | BE | |
| Kenya | 1997 | N/A | 247 | 5.3% | N/A | Ogutu et al.41 | N/A | C | Major private hospitals in Nairobi |
| Nigeria | 1985 | N/A | 603 | 1.9% | N/A | Ogunbiyi42 | 1.32 | C | |
| Nigeria | 2011 | N/A | 320 | 9.4% | 59.5 | Alatise et al.43 | 1.32 | C | |
| Asia | |||||||||
| Bangladesh | 2011 | Right-sided | 630 | 2.7% | 67.3 | Lahiri et al.44 | 1.38 | C | |
| India | 2002 | Left-sided | 225 | 4.4% | 54.8 | Kang et al.45 | 1.47 | C | Indians at St. George's Hospital, London |
| Iran | 1981 | N/A | 556 | > 20 y 1.6% > 50 y 2.4%, < 50 y 1.2% | N/A | Dabestani et al.46 | N/A | BE | |
| Japan | 2007 | Right-sided | 672 | 24.5% | 50 | Hirata et al.47 | 1.48 | C | |
| Japan | 2011 | Right-sided | 28.192 | 20.3% | 67.6 | Nagata et al.48 | 1.62 | C | |
| Japan | 2012 | Right-sided | 2164 | 25.1% | 58 | Nagata et al.49 | 1.36 | C | |
| Korea | 2008 | Right-sided | 848 | 12.1% | 50.9 | Song et al.28 | 1.58 | C | |
| Saudi Arabia | 2011 | Left-sided | 3649 | 7.4% | 60.8 | Azzam et al.50 | 1.57 | C | |
| Singapore | 2002 | Right-sided | 1663 | 45% | 59 | Fong et al.51 | 1.13 | BE | Chinese 84%, Indian 7%, Malay 6%, others 3% |
| Thailand | 2011 | Right-sided | 2877 | 28.5% | 59.8 | Lohsiriwat and Suthikeeree52 | 1.62 | BE | |
| America | |||||||||
| USA | 2005 | N/A | 542.868 | 42.8% | 50–59 | Everhart et al.53 | 0.84 | C | US-whites 86%, US-blacks 6%, US-Hispanics 5%, others 3% |
| USA | 2010 | Left-sided | 2104 | 41.7% | 53.9 | Peery et al.12 | 0.72 | C | US-whites 81%, US-non-whites 19% |
| Europe | |||||||||
| France | 2005 | Left-sided | 796 | 40.0% | 59.6 | Faucheron et al.54 | 1.16 | C | |
| Greece | 1999 | N/A | 502 | 22.9% | 65 | Paspatis et al.55 | 1.76 | A | |
| Netherlands | 2002 | N/A | 6827 | 27.0% | 56.6 | Loffeld and Van der Putten56 | 0.74 | C | |
| Poland | 2002 | Left-sided | 1912 | 21.7% | 55.4 | Blachut et al.57 | 0.56 | BE |
Updated and modified from Delvaux2 and Jun and Stollman.58 aFinal year of survey (if available) or date of submission. If available, the predominant location of diverticula is specified. As older studies found lower prevalence rates, only studies after 1990 were included (except for the studies from Iran, Kenya and Nigeria). Diagnostic modalities: A: autopsy; BE: barium enema; C: colonoscopy. M: male; F: female; y: year.
Europe and America
Traditionally, high prevalence rates for diverticulosis are reported for Europe and North America. This is based on two dated but repeatedly cited studies (29.7%19 and 18.3%59). Indeed, large recent epidemiological studies have confirmed these prevalence rates (27.0–42.8%).12,53,56
Africa
Mostly small series (maximum 20 patients) are available. Larger studies by Calder (226 patients),40 Ogunbiyi (603 patients),42 Ogutu et al. (247 patients)41 and Alatise et al. (320 patients)43 report low prevalence rates (1.9–9.4%), while newer prospective data suggest prevalence rates approximating those in Western countries.60
Asia
Whereas older studies found low prevalence rates (1.6–20.0%),46,61 longitudinal studies reported rising prevalence rates (8.4–45.0%),51,62 which is supported by newer series reporting a higher frequency (24.5%47 and 20.3%48).
The reasons for the differences in prevalence depending on ethnicity have yet to be studied systematically. They might be associated with differences in the level of exposure to specific environmental factors that contribute to the development of diverticulosis and DD across different world regions, such as the intake of fibre,13,14 red meat,14 vitamins,16 vegetarian diet13 or prevalence of obesity.28 Genetic factors contributing to the development of diverticulosis and DD are likely though.5,6
Migration studies
The distribution of DD and diverticulosis is often believed to be dictated by diet and environment. Migration studies are used to understand the influence of genetic and environmental factors in diseases whose incidence varies depending on ethnicity. Here, moving populations are assessed, and no change in disease incidence despite adopting the new environmental factors hints towards causal genetic factors. Turkish migrants in the Zaanstreek region (The Netherlands) have a significantly lower incidence of diverticulosis than the native Dutch population: For example, 7.5% of Turkish immigrants were found to have diverticulosis, in contrast to 50% of native Dutch.38 The predominant location of diverticula in Japanese living in Hawaii with Western living conditions and diet remains on the right side, indicating the role of genetic factors.39
Inherited diseases of the connective tissue
Several inherited diseases of the connective tissue are associated with diverticulosis and DD. This can be explained by the hypothesis that upon a weakness of the colonic wall, which is commonly affected in these diseases, aging and other environmental factors contribute to the development of diverticula. Large cross-sectional studies are not available though, and the molecular mechanisms are unknown. In this section we summarise the current evidence on the association of diverticulosis and DD with inherited diseases of the connective tissue.
EDS
EDS is a group of inherited connective tissue disorders caused by defects in collagen synthesis. The severity of the syndrome varies. EDS most typically affects the joints, skin, blood vessels and gastrointestinal tract. Major signs are hyperelasticity of the skin and hypermobility of the joints. EDS type IV (Online Mendelian Inheritance in Man (OMIM) 130050) is an autosomal-dominant defect of type-III collagen synthesis due to COL3A1 mutations. The phenotype is characterised by a peculiar facial appearance and spontaneous rupture of the bowel and large arteries. Small case series finding diverticulosis at an early age (two cases each)63,64 and two larger studies finding 28% colonic complications65 and various intestinal complications in an older study66 suggest an increased prevalence of DD in EDS type IV.
Williams-Beuren syndrome
Williams-Beuren syndrome (OMIM 194050) is a rare neurodevelopmental disorder that is characterised by a distinctive facial appearance, developmental delay and cardiovascular malformations. It is caused by a deletion on chromosome 7q11.23. The deleted region includes more than 25 genes (CLIP2, ELN, GTF2I and LIMK1 are among the genes that are typically deleted). Gastrointestinal symptoms are among the most common findings of the syndrome, which includes gastro-oesophageal reflux, constipation, rectal prolapse, hernias as well as the occurrence of DD at an early age in one series (prevalence 10.9% at mean age 26.9 years)67 and a case report of diverticulitis in a boy at the age of 9 years.68
Autosomal Dominant Polycystic Kidney Disease (ADPKD)
ADPKD results in cystic kidney dysfunction and can be caused by mutations in different genes: PKD1 (OMIM 173900), PKD2 (OMIM 613095) and PKD3 (OMIM 600666) cause a similar phenotype. Diverticulosis is a common finding in ADPKD, with one study finding a prevalence of 83%69 and another 75%.70 The diverticula are predominantly located in the right colon. The incidence and severity of diverticulitis is markedly increased in patients with ADPKD,71 hence even prophylactic colectomy before kidney transplantation has been recommended in individual cases. Although larger case series are needed, it seems reasonable to consider diverticulosis as an extrarenal manifestation of ADPKD.
Coffin-Lowry syndrome
Coffin-Lowry syndrome (OMIM 303600) is an X-chromosomal semi-dominant genetic disease that is caused by mutations in RPS6KA3, which encodes for ribosomal S6 kinase 2. This kinase has regulatory functions in skeletal and neural development. The syndrome is characterised by mental retardation that is commonly associated with auditory and visual abnormalities and kyphoscoliosis. Even though larger case series are missing, an autopsy case in siblings suggests that these patients might be prone to the development of diverticula.72
Marfan syndrome
Marfan syndrome is transmitted as an autosomal dominant trait and displays a broad phenotype. The most serious complications are afflictions of the heart valves and aorta. Other organs likely to be affected include the lungs, eyes and bone. Case reports suggest a possible higher frequency of diverticulosis in Marfan syndrome type 173,74 (OMIM 154700), but the prevalence of diverticulosis in Marfan syndrome is currently unknown; therefore, larger studies are needed.
Twin studies
Recently, two large twin studies calculated the heritability in DD. Twin studies take advantage of the fact that monozygotic twins share 100% of their genome, whereas dizygotic twins share about 50% of their genes. Twin studies compare the similarity of the phenotype of monozygotic and dizygotic twins. If the phenotype of monozygotic twins is significantly more similar than in dizygotic twins, this indicates the presence of influential genetic factors. Statistical calculations (structural equation modelling (SEM), tetrachoric correlation, ACE/A&E models) are used to estimate the heritability of DD. Box 2 defines the terminology used for twin studies in this review. Granlund et al.5 linked the Swedish Twin Registry to the Swedish Inpatient Registry. A total of 104,452 twins born between 1886 and 1980 and not dead before 1969 were included, among whom 2296 presented with DD. The OR of developing the disease given one's co-twin was affected was 7.15 (95% confidence interval (CI) = 4.82–10.61) for monozygotic twins and 3.20 (95% CI = 2.21–4.63) for same-gender dizygotic twins. Accordingly, concordance rates (0.12% vs 0.06%) and tetrachoric correlations (0.43% vs 0.21%) were significantly higher in monozygotic compared to dizygotic twins. Using SEM and the AE model,75 the heritability was estimated to be 40% and the non-shared environmental effects were calculated to account for 60% of the trait variability (Figure 1(a)). The Danish twin study,6 using the Danish National Registry of Patients linked to the Danish Twin Registry, included 30,322 twins born between 1870 and 2010 who were alive and living in Denmark in 1977. Among them 923 twins presented with DD. The relative risk for diverticulosis in siblings of index cases was 2.92 (95% CI = 2.50–3.39) as compared with the general population. The heritability (AE model) was estimated to be 53% (95% CI = 45–61%) (Figure 1(b)). In conclusion, these two population-based twin studies provide evidence that genetic factors contribute significantly to susceptibility for DD.
Box 2.
Statistical genetics for twin studies
| ACE/A&E model |
| Model to estimate the proportion of variation in trait susceptibility attributable to shared genetic effects (A), shared environmental effects (C) as well as non-shared, i.e. individual environmental effects (E); if there is no significant reduction in fit of the restricted model, the C parameter can be dropped, resulting in estimates of A and E (A&E model) |
| Heritability |
| Proportion of the total phenotypic variance of a trait that can be attributed to genetic effects |
| Structural equation modelling (SEM) |
| Method to estimate the proportion of variation of shared genetic and environmental effects on a phenotype as the contribution of unmeasured (latent) variables to the potentially multivariate phenotypic differences |
| Tetrachoric correlation |
| Measure assuming an underlying binormal distribution of susceptibility with multiple factors contributing additively and a threshold value that differs as presence or absence of the trait; higher tetrachoric correlation in monozygotic compared to dizygotic twins indicates the presence of genetic effects |
Figure 1.
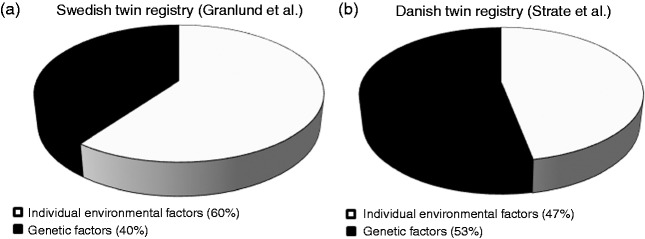
Pie diagrams depicting the contribution of genetic and environmental factors to the development of diverticulosis in twins. SEM was employed to estimate the contributions of the factors to the phenotypic variation observed among 2296 twins with DD in the Swedish Twin Registry (primary and secondary diagnoses, Figure 1(a)) and 923 twins with DD in the Danish Twin Registry (Figure 1(b)) assuming AE models. SEM: structural equation modelling; DD: diverticular disease; A: genetic effects; E: individual environmental factors.
Family and linkage studies
Currently no large family studies are available. Several case reports describe the occurrence of familial diverticulosis and DD: Cases of severe sigmoid diverticulitis in monozygotic twins were presented by Pusch et al.76 and Frieden and Morgenstern.77 Early occurrence of diverticulosis was described in three Nigerian siblings,78 three sisters in Italy,79 two young adult brothers80 and two siblings under the age of 20.81
Association studies
Association studies are widely used to assess the role of common genetic variants in complex traits.82 They compare the frequencies of genetic variants between cases and controls. Though data from association studies in diverticulosis and DD are scarce, only a small candidate-gene study with only 26 patients reported a single nucleotide polymorphism (SNP) in TNFSF15 (rs7848647),83 which was associated with diverticulitis requiring surgical therapy. Polymorphisms in TNFSF15 are associated with inflammatory bowel disease (particularly with a severe phenotype in both ulcerative colitis and Crohn's disease) as well as pouchitis. The cytokine TL1A, which is encoded by TNFSF15, belongs to the tumour necrosis factor (TNF) ligand family and thus might mediate inflammation in DD. The study, however, assessed surgically treated diverticulitis only and, because of the small sample size, the findings remain inconclusive for diverticulosis and DD. Another candidate-gene study (n = 52) assessing colon cancer risk variants coincidentally found an association between diverticulosis and a variant in RPRM,84 with SNP frequencies deviating from Hardy-Weinberg equilibrium in patients with DD. RPRM is a tumour suppressor gene and involved in the regulation of p53-mediated G2 arrest of the cell cycle. In line with the hypothesis that diverticula are protrusions through vulnerable locations in connective tissue, genetic polymorphisms predisposing to other protrusions in the connective tissue have been identified in larger series in DD-related diseases such as in hiatus hernia85 or pelvic prolapse.86
Monogenic diverticulosis/DD
Currently, no case of monogenic DD is known, and no causative mutations could be identified.
Conclusions
DD is a common disease characterised by considerable inter-individual variability in terms of severity and morbidity. The spectrum of the disease ranges from the asymptomatic presence of diverticula to severe complications such as perforation requiring emergency surgery. Diverticulosis and DD with its different stages have distinct risk factors, both genetically and environmentally. Diverticulitis and its complications are caused by the onset of inflammation, which represents a pathomechanism not involved in the diverticula in diverticulosis. Most risk factors for the specific conditions have not been defined yet. Inherited diseases of the connective tissue, such as EDS, PKD and Williams-Beuren syndrome, are associated with diverticulosis. Different ethnicities sharing similar living conditions display distinct prevalence rates, and migration into Western countries appears to have no major effects on the phenotype of diverticulosis and DD. Recently the heritability as calculated in twin studies has provided conclusive evidence that genetic factors contribute to DD susceptibility. Even though these results have to be proven for asymptomatic diverticulosis, DD and diverticulosis should be considered as a complex genetic disease resulting from environmental factors interacting with multiple susceptibility genes and disease modifiers. The multifactorial aetiology appears to be the most challenging task in identifying the disease-causing mechanisms. Once identified, susceptibility genes may allow preventive screening and development of novel therapeutic options.
To identify predisposing genetic polymorphisms, improvements in sequencing technologies allow the development of high throughput, parallel sequencing.87 Genomewide association studies (GWAS) have had great success in identifying genetic loci for complex diseases such as type 2 diabetes, rheumatoid arthritis, obesity and Crohn's disease. GWAS have not been conducted in diverticulosis and DD at this point and are urgently required to identify common risk variants. By focusing on transcribed gene regions, time and effort can be reduced by exome sequencing, but this approach spares noncoding regions, which might also be of interest. Thus this strategy might serve as a first step, and could be further extended to genomic analysis of structural variation, epigenetic modifications and transcriptomics of DD in the future. Collaboration within large consortia and multidisciplinary efforts are necessary to shed light on causative genetic variation in diverticulosis and DD. As the costs are continuously dropping, (sequencing-based) GWAS are likely to identify common “diverticulogenic” genes in future. The discovery of these genes might provide opportunities to control the influence of specific environmental factors. This could also lead to the development of interventions that extend our currently limited knowledge for the prevention and therapy of this exceptionally prevalent digestive disease.
Acknowledgements
The authors would like to thank Caroline Stokes, PhD, for critical editing and Paul Feuerstadt, MD, FACG, for insightful comments on the manuscript.
Funding
This work was supported by a grant from the Faculty of Medicine, Saarland University (HOMFOR) to Matthias C Reichert.
Conflict of interest
None declared.
References
- 1.Peery AF, Dellon AS, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 Update. Gastroenterology 2012; 143: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delvaux M. Diverticular disease of the colon in Europe: Epidemiology, impact on citizen health and prevention. Aliment Pharmacol Ther 2003; 3: 71–74. [DOI] [PubMed] [Google Scholar]
- 3.Cohen E, Fuller G, Bolus R, et al. Increased risk for irritable bowel syndrome after acute diverticulitis. Clin Gastroenterol Hepatol 2013; 11: 1614–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahedi K, Fuller G, Bolus R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol 2013; 11: 1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granlund J, Svensson T, Olén O, et al. The genetic influence on diverticula disease – a twin study. Aliment Pharmacol Ther 2012; 35: 1103–1107. [DOI] [PubMed] [Google Scholar]
- 6.Strate LL, Erichsen R, Baron JA, et al. Heritability and familial aggregation of diverticular disease: A population-based study of twins and siblings. Gastroenterology 2013; 144: 736–742. [DOI] [PubMed] [Google Scholar]
- 7.Painter NS, Burkitt DP. Diverticular disease of the colon: A deficiency disease of Western civilization. Br Med J 1971; 22: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manousos O, Day NE, Tzonou A, et al. Diet and other factors in the aetiology of diverticulosis: An epidemiological study in Greece. Gut 1985; 26: 544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodribb AH, Humphreys DM. Diverticular disease: Three studies. Part I – Relation to other disorders and fibre intake. Br Med J 1976; 1: 424–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr 1998; 128: 714–719. [DOI] [PubMed] [Google Scholar]
- 11.Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol 2013; 11: 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against symptomatic diverticulosis. Gastroenterology 2012; 142: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldoori WH, Giovannucci EL, Rimm EB, et al. A prospective study of diet and the risk of symptomatic diverticular disease in men. Am J Clin Nutr 1994; 60: 757–764. [DOI] [PubMed] [Google Scholar]
- 14.Crowe FL, Appleby PN, Allen NE, et al. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): Prospective study of British vegetarians and non-vegetarians. BMJ 2011; 343: d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowe FL, Balkwill A, Cairns BJ, et al. Source of dietary fibre and diverticular disease incidence: A prospective study of UK women. Gut 2014; 63: 1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maguire L, Song M, Strate LE, et al. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol 2013; 11: 1631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maguire LH, Song M, Strate LL, et al. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg 2014; 150: 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stokes CS, Volmer DA, Grünhage F, et al. Vitamin D in chronic liver disease. Liver Int 2013; 33: 338–352. [DOI] [PubMed] [Google Scholar]
- 19.Gear JS, Ware A, Fursdon P, et al. Symptomless diverticular disease and intake of dietary fibre. Lancet 1979; 1: 511–514. [DOI] [PubMed] [Google Scholar]
- 20.Lin OS, Soon MS, Wu SS, et al. Dietary habits and right-sided colonic diverticulosis. Dis Colon Rectum 2000; 43: 1412–1418. [DOI] [PubMed] [Google Scholar]
- 21.Strate LL, Liu YL, Aldoori WH, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA 2008; 300: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hjern F, Wolk A, Hakansson N. Smoking and the risk of diverticular disease in women. Br J Surg 2011; 98: 997–1002. [DOI] [PubMed] [Google Scholar]
- 23.Turunen P, Wikström H, Carpelan-Holmström, et al. Smoking increases the incidence of complicated diverticular disease of the sigmoid colon. Scand J Surg 2010; 99: 14–17. [DOI] [PubMed] [Google Scholar]
- 24.Usai P, Ibba I, Lai M, et al. Cigarette smoking and appendectomy: Effect on clinical course of diverticulosis. Dig Liver Dis 2011; 43: 98–101. [DOI] [PubMed] [Google Scholar]
- 25.Strate LL, Liu YL, Aldoori WH, et al. Physical activity decreases diverticular complications. Am J Gastroenterol 2009; 104: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hjern F, Wolk A, Hakansson N. Obesity, physical inactivity, and colonic diverticular disease requiring hospitalization in women: A prospective cohort study. Am J Gastroenterol 2012; 107: 296–302. [DOI] [PubMed] [Google Scholar]
- 27.Rosemar A, Angeras U, Rosengren A. Body mass index and diverticular disease: A 28-year follow-up study in men. Dis Colon Rectum 2008; 51: 450–455. [DOI] [PubMed] [Google Scholar]
- 28.Strate LL, Liu YL, Aldoori WH, et al. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology 2009; 136: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song JH, Kim YS, Lee JH, et al. Clinical characteristics of colonic diverticulosis in Korea: A prospective study. Korean J Intern Med 2010; 25: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopylov U, Ben-Horin S, Lahat A, et al. Obesity, metabolic syndrome and the risk of development of colonic diverticulosis. Digestion 2012; 86: 201–205. [DOI] [PubMed] [Google Scholar]
- 31.Humes DJ, Solaymani-Dodaran M, Fleming KM, et al. A population-based study of perforated diverticular disease incidence and associated mortality. Gastroenterology 2009; 136: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 32.Jansen A, Harenberg S, Grenda U, et al. Risk factors for colonic diverticular bleeding: A westernized community based hospital study. World J Gastroenterol 2009; 15: 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hjern F, Mahmood MW, Abraham-Nordling M, et al. Cohort study of corticosteroid use and risk of hospital admission for diverticular disease. Br J Surg 2014; 102: 119–124. [DOI] [PubMed] [Google Scholar]
- 34.Kvasnovsky CL, Papagrioriadis S, Bjarnason I. Increased diverticular complications with NSAIDs and other medications: A systematic review and meta-analysis. Colorectal Dis 2014; 16: 189–196. [DOI] [PubMed] [Google Scholar]
- 35.Strate LL, Liu YL, Huang ES, et al. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology 2011; 140: 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajendra S, Ho JJ. Colonic diverticular disease in a multiracial Asian patient population has an ethnic predilection. Eur J Gastroenterol Hepatol 2005; 17: 871–875. [DOI] [PubMed] [Google Scholar]
- 37.Lee YS. Diverticular disease of the large bowel in Singapore. An autopsy survey. Dis Colon Rectum 1986; 29: 330–335. [DOI] [PubMed] [Google Scholar]
- 38.Loffeld RJ. Diverticulosis of the colon is rare amongst immigrants living in the Zaanstreek region in the Netherlands. Colorectal Dis 2005; 7: 559–562. [DOI] [PubMed] [Google Scholar]
- 39.Stemmermann GN. Patterns of disease among Japanese living in Hawaii. Arch Environ Health 1970; 20: 266–273. [DOI] [PubMed] [Google Scholar]
- 40.Calder JF. Diverticular disease of the colon in Africans. Br Med J 1979; 6176: 1465–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogutu EO, Okoth FA, Lule GN. Colonoscopic findings in Kenyan African patients. East Afr Med J 1998; 75: 540–543. [PubMed] [Google Scholar]
- 42.Ogunbiyi OA. Diverticular disease of the colon in Ibadan, Nigeria. Afr J Med Med Sci 1989; 18: 241–244. [PubMed] [Google Scholar]
- 43.Alatise OI, Arigbabu AO, Agbakwuru EA, et al. Spectrum of colonoscopy findings in Ile-Ife Nigeria. Niger Postgrad Med J 2012; 19: 219–224. [PubMed] [Google Scholar]
- 44.Lahiri RP, Abeles A, Burnand KM, et al. A cross sectional study of colonic diverticulosis in the London Bangladeshi population. United European Gastroenterol J 2013; 1: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang JY, Dhar A, Pollok R, et al. Diverticular disease of the colon: Ethnic differences in frequency. Aliment Pharmacol Ther 2004; 19: 765–769. [DOI] [PubMed] [Google Scholar]
- 46.Dabestani A, Aliabadi P, Shah-Rookh FD, et al. Prevalence of colonic diverticular disease in southern Iran. Dis Colon Rectum 1981; 24: 385–387. [DOI] [PubMed] [Google Scholar]
- 47.Hirata T, Kawakami Y, Kinjo N, et al. Association between colonic polyps and diverticular disease. World J Gastroenterol 2008; 14: 2411–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagata N, Niikura R, Aoki T, et al. Increase in colonic diverticulosis and diverticular hemorrhage in an aging society: Lessons from a 9-year colonoscopic study of 28,192 patients in Japan. Int J Colorectal Dis 2014; 29: 379–385. [DOI] [PubMed] [Google Scholar]
- 49.Nagata N, Niikura R, Shimbo T, et al. Alcohol and smoking affect risk of uncomplicated colonic diverticulosis in Japan. PLoS One 2013; 8: e81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azzam N, Aljebreen AM, Alharbi O, et al. Prevalence and clinical features of colonic diverticulosis in a Middle Eastern population. World J Gastrointest Endosc 2013; 16: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fong SS, Tan EY, Foo A, et al. The changing trend of diverticular disease in a developing nation. Colorectal Dis 2011; 13: 312–316. [DOI] [PubMed] [Google Scholar]
- 52.Lohsiriwat V, Suthikeeree W. Pattern and distribution of colonic diverticulosis: Analysis of 2877 barium enemas in Thailand. World J Gastroenterol 2013; 19: 8709–8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology 2009; 136: 741–754. [DOI] [PubMed] [Google Scholar]
- 54.Faucheron JL, Roblin X, Bichard P, et al. The prevalence of right-sided colonic diverticulosis and diverticular haemorrhage. Colorectal Dis 2013; 15: 266–270. [DOI] [PubMed] [Google Scholar]
- 55.Paspatis GA, Papanikolaou N, Zois E, et al. Prevalence of polyps and diverticulosis of the large bowel in the Cretan population. An autopsy study. Int J Colorectal Dis 2001; 16: 257–261. [DOI] [PubMed] [Google Scholar]
- 56.Loffeld RJ, Van der Putten AB. Diverticular disease of the colon and concomitant abnormalities in patients undergoing endoscopic evaluation of the large bowel. Colorectal Dis 2002; 4: 189–192. [DOI] [PubMed] [Google Scholar]
- 57.Blachut K, Paradowski L, Garcarek J. Prevalence and distribution of the colonic diverticulosis. Review of 417 cases from Lower Silesia in Poland. Rom J Gastroenterol 2004; 13: 281–285. [PubMed] [Google Scholar]
- 58.Jun S, Stollman N. Epidemiology of diverticular disease. Best Pract Res Clin Gastroenterol 2002; 16: 529–542. [DOI] [PubMed] [Google Scholar]
- 59.Manousos ON, Truelove SC, Lumsden K. Prevalence of colonic diverticulosis in general population of Oxford area. BMJ 1967; 3: 762–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alatise OI, Arigbabu AO, Lawal OO, et al. Presentation, distribution ,pattern, and management of diverticular disease in a Nigerian tertiary hospital. Niger J Clin Pract 2013; 16: 226–231. [DOI] [PubMed] [Google Scholar]
- 61.Chia JG, Wilde CC, Ngoi SS, et al. Trends of diverticular disease of the large bowel in a newly developed country. Dis Colon Rectum 1991; 34: 498–501. [DOI] [PubMed] [Google Scholar]
- 62.Munakata A, Nakaji S, Takami H, et al. Epidemiological evaluation of colonic diverticulosis and dietary fiber in Japan. Tohoku J Exp Med 1993; 171: 145–151. [DOI] [PubMed] [Google Scholar]
- 63.Bläker H, Funke B, Hausser I, et al. Pathology of the large intestine in patients with vascular type Ehlers-Danlos syndrome. Virchows Arch 2007; 450: 713–717. [DOI] [PubMed] [Google Scholar]
- 64.Lindor NM, Bristow J. Tenascin-X deficiency in autosomal recessive Ehlers-Danlos syndrome. Am J Med Genet A 2005; 135: 75–80. [DOI] [PubMed] [Google Scholar]
- 65.Pepin M, Schwarze U, Superti-Furga A, et al. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. New Engl J Med 2000; 342: 673–680. [DOI] [PubMed] [Google Scholar]
- 66.Beighton PH, Murdoch JL, Votteler T. Gastrointestinal complications of the Ehlers-Danlos syndrome. Gut 1969; 10: 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Partsch CJ, Siebert R, Caliebe A, et al. Sigmoid diverticulitis in patients with Williams-Beuren syndrome: A relatively high prevalence and high complication rate in young adults with the syndrome. Am J Med Genet A 2005; 137: 52–54. [DOI] [PubMed] [Google Scholar]
- 68.Ignacio RC, Klapheke WP, Stephen T, et al. Diverticulitis in a child with Williams syndrome: A case report and review of the literature. J Pediatr Surg 2012; 47: 33–35. [DOI] [PubMed] [Google Scholar]
- 69.Scheff RT, Zuckerman G, Harter H, et al. Diverticular disease in patients with chronic renal failure due to polycystic kidney disease. Ann Intern Med 1980; 92: 202–204. [DOI] [PubMed] [Google Scholar]
- 70.Pourfarziani V, Mousavi-Nayeeni SM, Ghaheri H, et al. The outcome of diverticulosis in kidney recipients with polycystic kidney disease. Transplant Proc 2007; 39: 1054–1056. [DOI] [PubMed] [Google Scholar]
- 71.Lederman ED, McCoy G, Conti DJ, et al. Diverticulitis and polycystic kidney disease. Am Surg 2000; 66: 200–203. [PubMed] [Google Scholar]
- 72.Machin GA, Walther GL, Fraser VM. Autopsy findings in two adult siblings with Coffin-Lowry syndrome. Am J Med Genet Suppl 1987; 3: 303–309. [DOI] [PubMed] [Google Scholar]
- 73.Eliashar R, Sichel JY, Biron A, et al. Multiple gastrointestinal complications in Marfan syndrome. Postgrad Med J 1998; 74: 495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suster SM, Ronnen M, Bubis JJ. Diverticulosis coli in association with Marfan's syndrome. Arch Intern Med 1984; 144: 203. [PubMed] [Google Scholar]
- 75.Falconer DS, Mackay TFC. Introduction to quantitative genetics, New York: Longman Scientific & Technical, 1996. [Google Scholar]
- 76.Pusch HH, Börger G, Hirth L, et al. Perforating sigmoid diverticulitis in monozygotic twins [article in German]. MMW Münch Med Wochenschr 1979; 121: 947–948. [PubMed] [Google Scholar]
- 77.Frieden JH, Morgenstern L. Sigmoid diverticulitis in identical twins. Dig Dis Sci 1985; 30: 182–183. [DOI] [PubMed] [Google Scholar]
- 78.Omojola MF, Mangete E. Diverticula of the colon in three Nigerian siblings. Trop Geog Med 1988; 40: 54–57. [PubMed] [Google Scholar]
- 79.Vigoni A, Marcato M, Lo Monaco GP, et al. Diverticulosis and diverticular disease (reference to a case of diverticular disease observed in 3 sisters) [article in Italian]. Chir Ital 1985; 37: 656–659. [PubMed] [Google Scholar]
- 80.Graininger R, Edwards MH. Complicated colonic diverticular disease in two young adult brothers. J R Coll Surg Edinb 1987; 32: 255–256. [PubMed] [Google Scholar]
- 81.Claassen AT, Mourad-Baars PE, Mearin ML, et al. Two siblings below the age of 20 years with diverticular disease. Int J Colorectal Dis 2006; 21: 190–191. [DOI] [PubMed] [Google Scholar]
- 82.Krawczyk M, Müllenbach R, Weber SN, et al. Genome-wide association studies and genetic risk assessment of liver diseases. Nat Rev Gastroenterol Hepatol 2010; 12: 669–681. [DOI] [PubMed] [Google Scholar]
- 83.Connelly TM, Berg AS, Hegarty JP, et al. The TNFSF15 gene single nucleotide polymorphism rs7848647 is associated with surgical diverticulitis. Ann Surg 2014; 259: 1132–1137. [DOI] [PubMed] [Google Scholar]
- 84.Beasley WD, Beynon J, Jenkins GJ, et al. Reprimo 824 G>C and p53R2 4696 C>G single nucleotide polymorphisms and colorectal cancer: A case-control disease association study. Int J Colorectal Dis 2008; 23: 375–381. [DOI] [PubMed] [Google Scholar]
- 85.Åsling B, Jirholt J, Hammond P, et al. Collagen type III alpha I is a gastro-oesophageal reflux disease susceptibility gene and a male risk factor for hiatus hernia. Gut 2009; 58: 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kluiveres KB, Dijkstra JR, Hendriks JC, et al. COL3A1 2209 G>A is a predictor of pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2009; 20: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 87.Eid J, Fehr A, Gray J, et al. Real-time DNA sequencing from single polymerase molecules. Science 2009; 323: 133–138. [DOI] [PubMed] [Google Scholar]


