Fig. 2.
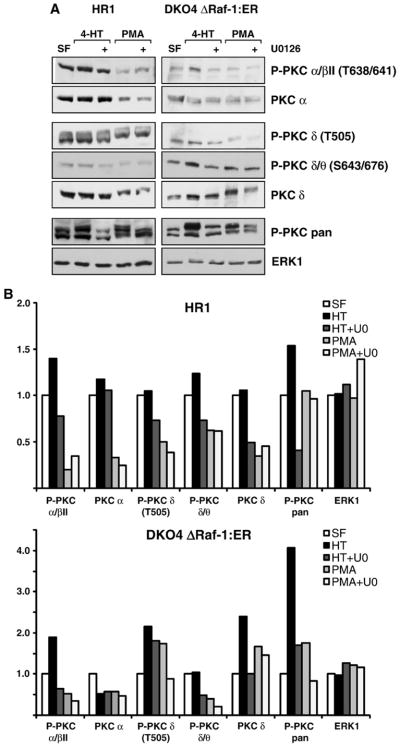
The crosstalk between ERK and PKC involves different PKC isoforms and mechanisms. (A) HR1 and DKO4 ΔRaf-1:ER cells were treated with PMA or 4-HT with or without U0126 in serum-free medium for 4 h. Whole-cell extracts were fractionated by SDS-PAGE and subjected to Western blotting with antibodies to P-PKC pan, to several different phospho-specific isoforms of PKC as indicated, to the non-phosphorylated isoforms PKC α and PKC δ, and to total ERK1. In both cell lines treatment with 4-HT or PMA caused a stimulation of P-PKC pan as seen before, and U0126 treatment partially reversed this P-PKC activation. Whereas the phospho-specific PKC isoform βII was regulated in a MEK/ERK-dependent manner the total levels of the PKC α subunit remained constant suggesting a change in stoichiometry of phosphorylation of PKC α or βII. Phospho-PKC δ also displayed MEK/ERK-dependent regulation, however, as the total levels of PKC δ were also affected, the differential PKC δ phosphorylation might be explained by auto-phosphorylation or changes in abundance. (B) Densitometric analysis of the Western blots shown in (A), the intensities are relative to the serum-free sample which was set to 1 in each case. Results are shown from single experiments representative of three in each case.
