Fig. 8.
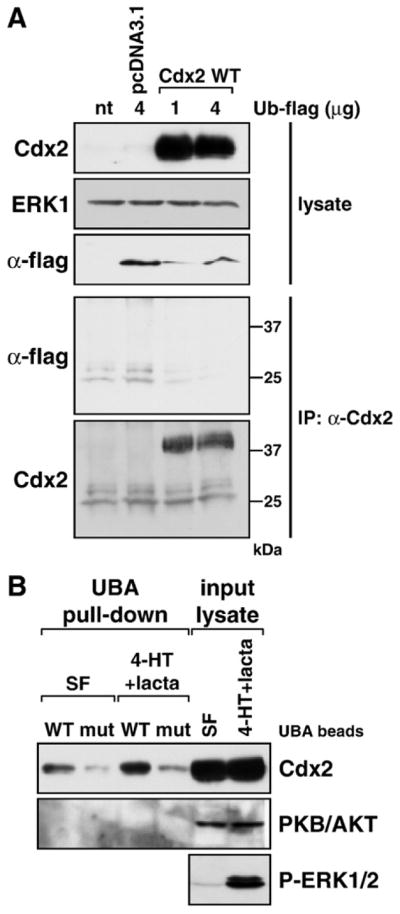
Ubiquitination of Cdx2 in response to ERK1/2 activation detected by binding to an immobilised UBA domain. (A) HR1 cells were transfected with wild-type Cdx2 or the control vector pcDNA3.1 in addition to a flag-tagged ubiquitin expression construct, and following overnight incubation were treated with the very potent proteasome inhibitor lactacystin (lacta) for 6 h. Expression of transfected Cdx2 and ubiquitin (α-flag) proteins was demonstrated in whole-cell lysates, ERK1 served as a loading control. Following immunoprecipitation of Cdx2 we were unable to detect extensive ubiquitination of Cdx2 in the pulled-down fraction (α-flag), even though Cdx2 was pulled down very nicely (α-Cdx2). (B) HR1 cells were transfected with wild-type Cdx2 and kept in either serum-free medium or in medium containing 4-HT plus lactacystin for 6 h to promote Cdx2 phosphorylation and ubiquitination as well as inhibit its proteasomal degradation. Western blots demonstrated that transfected Cdx2 was expressed well and that 4-HT+ lacta treatment activated the MEK/ERK1/2 pathway. Ubiquitinated proteins were pulled down using either wild-type or mutated UBA beads (designated WT or mut) and the pulled-down fraction was subjected to Western blotting against Cdx2 or protein kinase B (PKB/AKT). The amount of UBA-bound Cdx2 was increased upon 4-HT+lacta treatment compared to the serum-free sample. Endogenous PKB/AKT was not pulled down by UBA beads under any condition and served as a specificity control.
