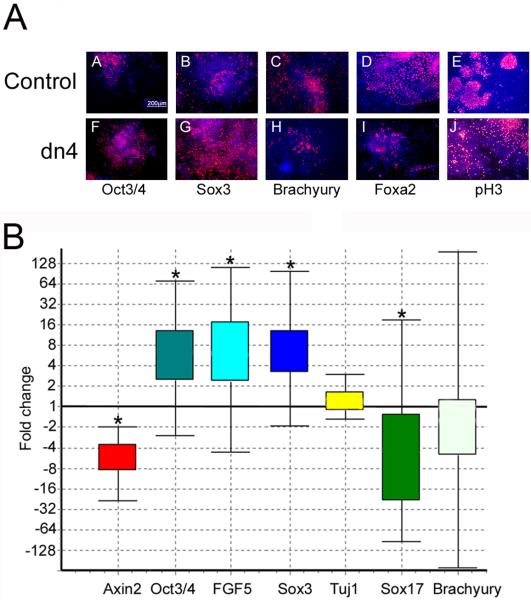
Figure 5. Wnt signaling blockade increases differentiation of neural precursors and decreases mesendoderm differentiation in an EB assay.
A. Control and dnTCF4 ESC were grown as EB for 4 days then transferred to adherent culture for an additional 2 days. Cells were grown without Dox during the entire culture period to induce transgene expression. IHC localization of the pluripotency marker (Oct3/4; A,F), the neural precursor marker (Sox3; B,G), mesodermal marker (Brachyury; C,H), or the endodermal maker (Foxa2; D,I), or phosphohistone3 to identify dividing cells (pH3; E,J). When Wnt signaling is inhibited, cells continue to express high levels of Oct3/4 and Sox3 (F,G) with a concomitant decrease in mesendoderm differentiation (H,I), compared with controls (A-D). Panels E,J illustrate the very similar pattern of cell division identified using anti-phosphohistone3 antibody. Secondary antibodies were conjugated to Cy3 (red) and Hoechst (blue) identifies nuclei.
B. Quantitative PCR indicated that expression of the Wnt target gene Axin2 was decreased 5-fold in dnTcf4 compared to control cells after 6 days of transgene induction. Genes expressed in the epiblast: Oct3/4 and Fgf5 were increased 6 fold suggesting that Wnt signaling is required for differentiation of the epiblast. The neural precursor gene Sox3 was increased 6 fold, but Tuj1, a marker of immature neurons, was not changed. Sox17 was down-regulated 5 fold and there was a trend towards a reduction of Brachyury expression (mesoderm) when Wnt signaling was inhibited. Box and whisker plot produced with (REST software, Pfaffl et al., 2002). The top and bottom “whiskers” indicate the range while the box indicates the upper and lower quartile values. Gene expression was calculated relative to β-actin and gene expression in the control ESC line was set to 1. Asterisks indicate statistically significant differences (p≤0.05) calculated by the REST software (n=3).