Abstract
Background
Limited data exist regarding toxicity and quality of life (QOL) after post-prostatectomy intensity modulated radiation therapy (IMRT) and whether pelvic nodal RT influences these outcomes.
Methods
118 men were treated with curative-intent RT after radical prostatectomy. 69 men (58%) received pelvic nodal RT. QOL data and physician-assigned toxicity were prospectively collected. Changes in QOL from baseline were assessed with Wilcoxon signed-rank tests and risk factors associated with each domain were identified with generalized estimating equation (GEE) models. Late freedom from (FF) toxicity was estimated by the Kaplan-Meier method and comparisons were tested using the log-rank test.
Results
Urinary irritation/obstruction, bowel, and sexual domain scores declined at 2 months (all P ≤ 0.01) but were no different than baseline at subsequent visits through 4 years of follow-up. At 4 years, FF grade 2+ GI toxicity was 90% and FF grade 2+ GU toxicity was 89%. On GEE analysis, pelvic nodal RT was associated with decreased bowel function (P = 0.09) and sexual function (P = 0.01). On multivariate analysis, however, there was no significant association with either decreased bowel (P = 0.31) or sexual (P = 0.84) function. There was also no association with either FF grade 2+ GI toxicity (P = 0.24) or grade 2+ GU toxicity (P = 0.51).
Conclusions
Receipt of pelvic nodal RT was not associated with inferior QOL or toxicity compared to prostate bed alone RT. For the entire cohort, RT was associated with only temporary declines in patient-reported urinary, bowel, or sexual QOL.
Introduction
Prostate cancer is the most common cancer in American men with 233,000 estimated new cases in 2014 [1]. Among men who receive treatment for localized prostate cancer, the majority undergo radical prostatectomy (RP) [2]. However, with certain high risk features, the risk of biochemical failure exceeds 50%. Radiation therapy (RT) after RP may be offered adjuvantly for adverse pathologic features [3–5] or for salvage after biochemical failure [6,7].
Notably, long term follow-up after post-prostatectomy radiation therapy (PPRT) suggests a moderate risk of relapse, even after a period of initial biochemical control. Although the role for intensification of PPRT by inclusion of the pelvic nodes has not been established prospectively, retrospective data demonstrate increased biochemical control in certain subsets of men [8,9]. However, the use of pelvic nodal RT in the post-operative setting may be tempered by concerns for increased toxicity. The primary goal of this study was to describe the influence of pelvic nodal RT on late toxicity and patient-reported quality of life (QOL) using a modern treatment approach including image guided, intensity modulated radiation therapy (IMRT). A secondary goal was to characterize QOL outcomes for the entire cohort of men treated with PPRT for use as a tool to guide patient expectations.
Methods
Between 2006 and 2012, 122 men consecutively treated with PPRT were identified from a prospectively collected database. This study was approved by the University of Chicago Institutional Review Board (IRB). The majority of men in this study provided written informed consent during ongoing follow-up visits. Waiver of informed consent due to the retrospective nature of this work was obtained for all other patients in whom follow-up was not ongoing and therefore written informed consent could not be obtained in a practical fashion. The University of Chicago IRB approved both the written informed consent procedure and waiver of informed consent in patients who could not provide written informed consent. All men were treated with curative intent and had no known metastatic disease at the time of RT. Four men who had fewer than two QOL assessments were excluded from the study, leaving 118 men in the study cohort. The majority of men (87%) received salvage RT for rising PSA after RP. Median time from RP to RT was 18 months (range 3–135) and median pre-RT PSA was 0.24 (range 0–6.7). Androgen deprivation therapy (ADT) was given to 66 men (56%) and was typically a combination of an anti-androgen and a luteinizing hormone-releasing hormone agonist. Hormone therapy could have been given prior to the start of RT though no men in the study were castrate-resistant. QOL measures were assessed using the Expanded Prostate Cancer Index Composite (EPIC-26) by patients at baseline (and prior to any neoadjuvant ADT), at 2-months after completing treatment, and at subsequent biannual follow-up visits. Patients who completed QOL surveys at follow-up but not at the time of consultation were included in the analysis to assess changes in QOL over time after completion of RT. Physician-rated toxicity scores (CTC v4) for gastrointestinal (GI) and genitourinary (GU) domains were also assigned prospectively at each follow-up visit.
All patients underwent computed tomography (CT) simulation in the supine position with upper and lower alpha cradles for custom immobilization. The bladder was drained and introduced with 120 cc of saline; patients were instructed to maintain a comfortably full bladder at the time of each treatment. IMRT was used for both prostate bed alone RT and whole pelvic RT. The prostate bed was contoured according to published guidelines [10] with 0.6–0.9 cm anisotropic expansions to PTV, or a uniform 5 mm expansion if surgical clips were used for image guidance [11]. Pelvic lymph node contours were defined according to Radiation Therapy Oncology Group guidelines with 7–8 mm expansions around pelvic blood vessels to clinical target volume and additional 7–8 mm expansions to planning target volume (PTV). Patients receiving prostate bed RT were treated to a median dose of 68 Gy (interquartile range 66–68 Gy); patients in the pelvic nodal RT group were treated to a median dose of 50.4 Gy to the pelvic nodes and 68.4 Gy to the prostate bed (interquartile range 66.6–68.4 Gy). Treatment planning goals prioritizing PTV coverage and rectal, bladder, penile bulb, and femoral head sparing were used. Greater than 99% of the PTV was covered by the 95% isodose line with no volume receiving greater than 110% of the prescription dose. The volume of rectum and bladder receiving 70 Gy, 65 Gy, and 40 Gy were limited to 20%, 40%, and 80% and 30%, 60%, and 80%, respectively. The volume of penile bulb and femoral heads receiving 50 Gy were limited to 50% and 10%, respectively. Image guidance for setup verification was performed with cone beam CT on the first and second treatment days to ensure proper soft tissue alignment as well as daily kilovoltage imaging aligning to bony anatomy or to surgical clips in the prostate bed when present.
Differences in baseline patient characteristics and treatment variables between the prostate bed alone and pelvic nodal RT groups were assessed by t-test or Mann-Whitney test, depending on the distributions for continuous variables, and by chi-square or Fisher’s exact test for categorical variables. Wilcoxon signed-rank tests were used to assess the changes in QOL measures from baseline at 2-, 6-, 12-, 18-, 24-, 36-, and 48-month follow-up. In the event that data from the 24-, 36-, or 48-month follow-up was missing but was available for the subsequent 30-, 42-, or 54-month follow-up, respectively, the QOL survey from the later time point was used. Patients who experienced biochemical recurrence after PPRT continued to follow-up in Radiation Oncology and complete QOL surveys. Generalized estimating equation (GEE) models were used to identify risk factors associated with each QOL domain over time, respectively. Models included the tested covariate and assessment time as independent variables, the baseline scores, and the interaction term between the covariate and time. Factors, including age, race, body-mass index (BMI), presence of diabetes mellitus (DM) as a comorbidity, current or prior tobacco use, androgen deprivation therapy (ADT) use, time from prostatectomy to RT, receipt of pelvic nodal RT, and RT dose were tested for an association with each global domain endpoint as part of GEE and multivariate analyses. Freedom from toxicity beyond 3 months was estimated by the Kaplan-Meier method and statistical comparisons were tested using the log-rank test.
Results
Baseline patient characteristics and treatment variables are presented in Table 1. Patients receiving pelvic nodal RT were significantly more likely to have a higher Gleason score, more advanced pathologic T stage, higher pre-RT PSA, lymph node positive disease, and receive ADT (all P ≤ 0.01). The percent of patients reporting specific levels of dysfunction or distress for each QOL domain at baseline and in follow-up is reported in Table 2. The median QOL scores over time for each domain are demonstrated graphically in Figs 1 and 2 and reported numerically below. GEE analyses for individual covariates and multivariate analyses were performed for each QOL domain and are shown in Table 3.
Table 1. Patient and treatment characteristics.
| Variable | Prostate bed alone RT | Pelvic nodal RT | P value |
|---|---|---|---|
| (N = 49) | (N = 69) | ||
| Mean age, years (SD) | 61.7 (6.9) | 62.3 (6.5) | 0.60 |
| Race–no. (%) | 0.47 | ||
| White | 32 (65.3) | 51 (73.9) | |
| African American | 14 (28.6) | 13 (18.8) | |
| Other | 3 (6.1) | 5 (7.2) | |
| Median BMI (range) | 29.8 (22.2–38.7) | 28.5 (19.0–57.4) | 0.57 |
| Diabetes mellitus–no. (%) | 3 (6.1) | 13 (18.8) | 0.06 |
| Gleason score–no. (%) | <0.01 | ||
| 6 | 8 (16.3) | 6 (8.7) | |
| 7 | 35 (71.4) | 34 (49.3) | |
| 8 | 2 (4.1) | 10 (14.5) | |
| 9 | 4 (8.2) | 19 (27.5) | |
| Pathologic T stage–no. (%) | 0.01 | ||
| pT2 | 21 (42.9) | 14 (20.3) | |
| pT3a | 20 (40.8) | 32 (46.4) | |
| pT3b | 7 (14.3) | 23 (33.3) | |
| pT4 | 1 (2.0) | 0 (0) | |
| Pathologic lymph node positive–no. (%) | 0 (0) | 14 (24.1) | <0.001 |
| Median pre-RT PSA, ng/mL (range) | 0.17 (0–0.87) | 0.28 (0.07–6.7) | <0.01 |
| ADT received–no. (%) | 3 (6.1) | 63 (91.3) | <0.001 |
| Median ADT duration, months (range) | 4 (3–5) | 4 (1–28) | 0.69 |
| Median RT dose, Gy (IQR) | 68 (66–68) | 68.4 (66.6–68.4) | 0.36 |
| Median time from RP to RT, months (range) | 16.5 (3–127) | 19 (3–135) | 0.80 |
| Median follow-up, months (range) | 38 (2–75) | 25 (4–76) | 0.17 |
Table 2. Percent of patients reporting specific levels of dysfunction or distress for each QOL domain.
| Variable | Baseline | 2 Mo | 6 Mo | 12 Mo | 18 Mo | 24 Mo | 36 Mo | 48 Mo |
|---|---|---|---|---|---|---|---|---|
| (N = 102) | (N = 100) | (N = 98) | (N = 93) | (N = 69) | (N = 64) | (N = 50) | (N = 33) | |
| Urinary function | ||||||||
| Irritation or obstruction | ||||||||
| Dysuria | 2 | 3 | 0 | 0 | 1 | 0 | 0 | 0 |
| Hematuria | 0 | 1 | 1 | 2 | 0 | 2 | 2 | 0 |
| Weak stream | 5 | 1 | 2 | 0 | 1 | 5 | 0 | 0 |
| Nocturia | 10 | 17 | 9 | 13 | 7 | 9 | 13 | 13 |
| Frequency | 7 | 10 | 5 | 5 | 7 | 5 | 12 | 6 |
| Incontinence | ||||||||
| Leaking ≥1 time per day | 49 | 37 | 36 | 35 | 36 | 39 | 39 | 36 |
| Frequent dribbling | 13 | 12 | 8 | 12 | 10 | 14 | 16 | 12 |
| Any pad use | 39 | 30 | 33 | 28 | 38 | 35 | 24 | 27 |
| Leaking problem | 12 | 15 | 7 | 14 | 12 | 6 | 17 | 15 |
| Overall urinary problem | 10 | 13 | 8 | 11 | 9 | 6 | 12 | 3 |
| Bowel function | ||||||||
| Urgency | 3 | 7 | 3 | 2 | 6 | 5 | 2 | 3 |
| Frequency | 1 | 5 | 3 | 1 | 4 | 2 | 0 | 0 |
| Fecal incontinence | 1 | 0 | 0 | 1 | 1 | 3 | 0 | 3 |
| Bloody stools | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 |
| Rectal pain | 3 | 5 | 1 | 0 | 1 | 3 | 0 | 0 |
| Overall bowel problem | 2 | 6 | 4 | 4 | 1 | 3 | 4 | 3 |
| Sexual function | ||||||||
| Poor erections | 69 | 70 | 64 | 67 | 58 | 65 | 63 | 66 |
| Difficulty with orgasm | 51 | 59 | 46 | 54 | 48 | 47 | 54 | 47 |
| Erections not firm | 69 | 78 | 68 | 77 | 65 | 77 | 70 | 76 |
| Erections not reliable | 63 | 71 | 65 | 67 | 58 | 72 | 64 | 66 |
| Sexually active | 33 | 32 | 33 | 30 | 38 | 28 | 37 | 37 |
| Poor sexual function | 62 | 68 | 63 | 65 | 60 | 66 | 56 | 72 |
| Overall sexuality problem | 44 | 31 | 42 | 42 | 24 | 27 | 33 | 28 |
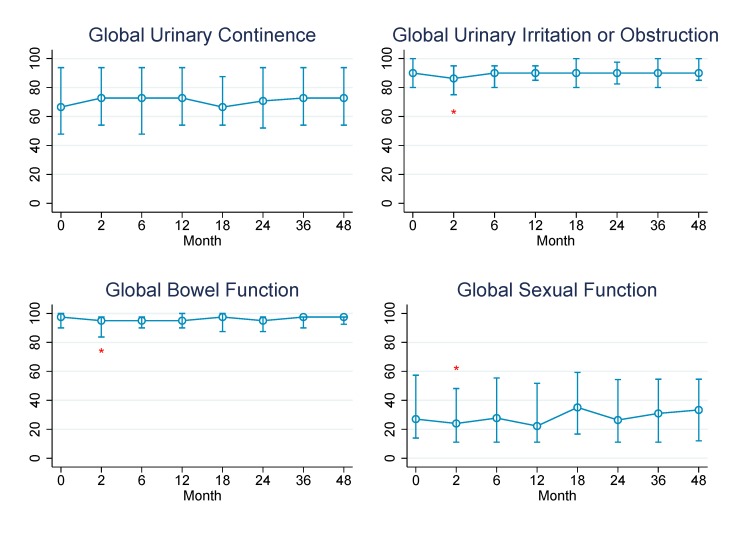
Fig 1. Changes in median QOL scores over time for the entire study cohort.
Asterisks designate time points at which scores were significantly worse than baseline (P < 0.05 by Wilcoxon signed-rank test). Standard error bars represent interquartile range.
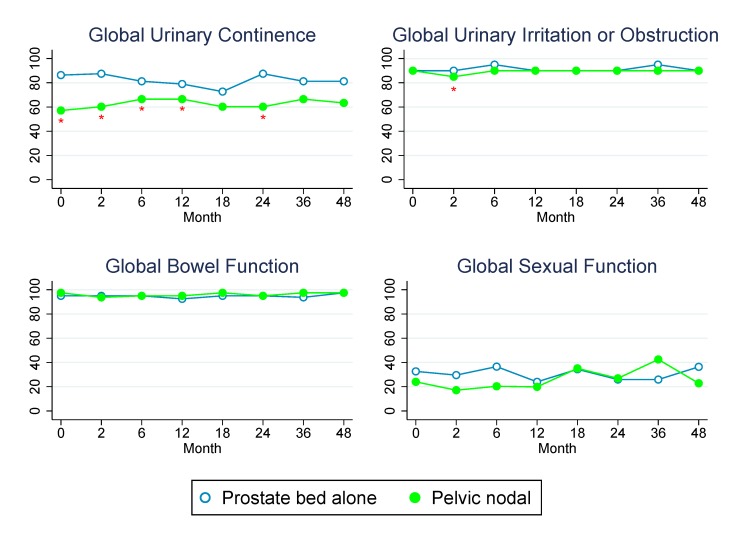
Fig 2. Changes in median QOL scores over time for patients treated with prostate bed versus pelvic nodal RT.
Asterisks designate time points at which scores were significantly different between groups (P < 0.05 by Mann-Whitney test). Standard error bars represent interquartile range.
Table 3. Factors associated with each QOL domain on multivariate analysis.
| QOL domain | Independent variable | P value |
|---|---|---|
| Urinary continence | Age | 0.73 |
| Race | ||
| African American vs. White | 0.56 | |
| Other vs. White | 0.34 | |
| BMI | 0.02 | |
| Diabetes mellitus | 0.37 | |
| Current tobacco use | 0.11 | |
| Prior tobacco use | 0.72 | |
| Pathologic T stage | 0.49 | |
| ADT use | 0.62 | |
| Time to RT | 0.31 | |
| Pelvic nodal RT | 0.84 | |
| RT dose | 0.75 | |
| Urinary irritation | Age | 0.31 |
| Race | ||
| African American vs. White | 0.70 | |
| Other vs. White | 0.69 | |
| BMI | 0.02 | |
| Diabetes mellitus | 0.52 | |
| Current tobacco use | 0.10 | |
| Prior tobacco use | 0.49 | |
| Pathologic T stage | 1.00 | |
| ADT use | 0.41 | |
| Time to RT | 0.72 | |
| Pelvic nodal RT | 0.32 | |
| RT dose | 0.04 | |
| Bowel function | Age | 0.49 |
| Race | ||
| African American vs. White | 0.002 | |
| Other vs. White | 0.67 | |
| BMI | 0.04 | |
| Diabetes mellitus | 0.80 | |
| Current tobacco use | 0.13 | |
| Prior tobacco use | 0.96 | |
| Pathologic T stage | 0.73 | |
| ADT use | 0.56 | |
| Time to RT | 0.77 | |
| Pelvic nodal RT | 0.31 | |
| RT dose | 0.52 | |
| Sexual function | Age | 0.05 |
| Race | ||
| African American vs. White | 0.15 | |
| Other vs. White | 0.82 | |
| BMI | 0.22 | |
| Diabetes mellitus | 0.27 | |
| Current tobacco use | 0.48 | |
| Prior tobacco use | 0.32 | |
| Pathologic T stage | 0.42 | |
| ADT use | 0.14 | |
| Time to RT | 0.95 | |
| Pelvic nodal RT | 0.84 | |
| RT dose | 0.40 |
Global urinary continence was unchanged from baseline through 4 years of follow-up at all time points (P > 0.1) whereas global urinary irritation/obstruction was significantly worse than baseline (median, 90.0) at 2 month follow-up (86.3, P = 0.001) but no different than baseline at 6 month follow-up (90.0) and subsequent visits (P = 0.84). At 4 years, freedom from grade 1+ or 2+ GU toxicity was 57% and 89%, respectively. Higher BMI was associated with worse urinary continence scores on GEE (P = 0.02) and multivariate (P = 0.02) analyses; no other factors were associated with urinary continence, including pelvic nodal RT. However, patients treated with pelvic nodal RT did have lower baseline global urinary continence scores, which was likely related to the more aggressive resection for these men with higher risk disease as described in Table 1. Higher BMI was also associated with worse urinary irritation/obstruction on GEE (P = 0.02) and multivariate (P = 0.02) analyses. When BMI was tested as a categorical variable on multivariate analysis, obesity (BMI ≥ 30) was associated with worse global urinary continence (P = 0.04) but not urinary irritation or obstruction (P = 0.11). Additionally, higher RT dose was associated with worse urinary irritation/obstruction on GEE (P = 0.06) and multivariate analysis (P = 0.04). There was no association between receipt of pelvic RT and worse urinary continence or irritation/obstruction scores on GEE (P = 0.45, P = 0.89) or multivariate analyses (P = 0.84, P = 0.32). There was also no association between receipt of pelvic RT and freedom from grade 1+ (P = 0.39) or grade 2+ (P = 0.51) GU toxicity.
Global bowel function was significantly worse than baseline (97.5) at 2 month follow-up (95.0, P < 0.001) but was no different than baseline at 6 month follow-up (95.0) and subsequent visits (P > 0.1). At 4 years, freedom from grade 1+ or 2+ GI toxicity was 66% and 90%, respectively. African-American race was associated with worse global bowel function on GEE (P = 0.004) and multivariate analyses (P = 0.002), as was higher BMI (P = 0.04). Obesity, however, was not associated with worse bowel or rectal function on multivariate modeling (P = 0.27). On GEE analysis, receipt of hormone therapy (P = 0.07) and receipt of pelvic nodal RT (P = 0.09) were weakly associated with worse bowel function; however, on multivariate analysis, there was no association (P = 0.56, P = 0.31). There was also no association between receipt of pelvic RT and freedom from physician-assigned grade 1+ (P = 0.64) or grade 2+ (P = 0.24) GI toxicity.
Global sexual function was also significantly worse than baseline (27.0) at 2 month follow-up (24.0, P = 0.01) but was no different than baseline at 6 month follow-up (27.7) and subsequent visits (P > 0.1 at all time points). On GEE analysis, increasing age (P = 0.04), receipt of pelvic nodal RT (P = 0.01), receipt of hormone therapy (P = 0.01), and the presence of DM as a comorbidity (P = 0.04) were all associated with worse global sexual function. On multivariate analysis, however, only increasing age was associated with worse sexual function (P = 0.05).
Discussion
In this cohort of men treated with PPRT who were prospectively evaluated with patient-reported QOL surveys and physician-rated toxicity, bladder, bowel, and sexual QOL remained stable through 4 years of follow-up, after only a transient decrease at 2 months. Additionally, the treatment of pelvic lymph nodes did not appear to negatively affect QOL or toxicity. Patients treated with pelvic nodal RT had higher risk disease, which likely contributed towards the relatively worse baseline urinary continence scores, but there was no disproportionate decline over time for men treated with pelvic nodal RT.
The negligible effect of pelvic nodal RT on toxicity in our series contrasts randomized data from the conventional (2D) RT era in the intact setting which demonstrated increased rates of late grade 2+ GU toxicity, and late grade 2 and 3+ GI toxicity with increasing field size for pelvic nodal coverage [12]. Our data are in line with other modern series which demonstrate reduced rates of acute [13] and late [14] GI toxicity with the use of IMRT in the post-prostatectomy setting. Our data also demonstrate stability with longer follow-up and more patients compared to an earlier analysis which showed no significant changes in urinary, bowel, or sexual QOL through 2 years of follow-up [15].
There are conflicting data regarding the toxicity of PPRT, perhaps related to differences in reporting and RT technique. In a patient-reported QOL analysis from the SWOG 8794 trial using a less detailed questionnaire than the EPIC-26, receipt of adjuvant RT was associated with worse bowel function through approximately 2 years after RT and worse urinary function through the reported 5 years of follow-up [16]. Toxicity data from the main trial also demonstrated high rates of urethral stricture and total urinary incontinence in patients receiving adjuvant RT, possibly because complications were published as crude percentages without regard to grade of toxicity or time of occurrence. In contrast, the EORTC 22911 trial demonstrated relatively low risks of severe acute and late toxicity with adjuvant RT. Additionally, a subset analysis demonstrated no difference in urinary continence as measured by pad weight between the group receiving adjuvant RT and the observation group [17]. In both of these trials, men were treated with conventional (2D) RT to 60–64 Gy. In a recent publication from Milan, receipt of dose-escalated adjuvant RT to a median dose of 70.2 Gy using 3D-conformal technique seemed to have a detrimental effect on recovery of urinary continence after RP [18]. In our series of mostly salvage PPRT to a median dose of 68 Gy using image-guided IMRT, global urinary continence scores demonstrated stability from baseline through 4 years of follow-up. Though global urinary irritation/obstruction, bowel function, and sexual function scores declined at 2 month follow-up, there was no difference from baseline on subsequent visits through 4 years of follow-up. Notably, RT dose was associated with urinary irritation/obstruction on multivariate modeling. It is possible that either the use of IMRT, the slightly lower dose prescribed in our series, or the generally delayed timing of RT mitigated the detrimental effect of PPRT on urinary function described in the Milan series.
It is interesting that higher BMI was associated with worse global urinary continence, urinary irritation/obstruction, and bowel or rectal function. Surgical series have demonstrated a similar association between obesity and delayed, less complete recovery of urinary [19,20] and bowel [21] function after RP, possibly due to the negative effect of obesity on the pelvic floor [22]. African-American race was also associated with worse global bowel or rectal function. Other series have similarly demonstrated worse urinary and bowel function in African-American men compared with white men after treatment of localized prostate cancer [23]; however, the small size of our series precludes us from drawing any firm conclusions from this result.
Though this analysis of prospectively collected data is limited by small sample size, it is among the few published series examining QOL after PPRT. It is reassuring to see no declines in QOL except for a transient decrease at 2 month follow-up, but it is theoretically possible that QOL scores could have continued to improve from baseline in the absence of PPRT. Given the median time from RP to RT in our series was 18 months, it is likely that bowel and bladder recovery of most patients had plateaued based on previously published QOL data after RP [24].
The benefit of pelvic nodal radiation for subsets of men in the post-prostatectomy setting has been suggested in retrospective series but awaits prospective validation. This is currently being investigated in RTOG 0534, which randomizes patients with rising PSA after RP to receive either prostate bed radiation with or without pelvic nodal RT. Until these data become available, our current institutional practice is to consider inclusion of the pelvic nodes in combination with ADT for select men with high-risk features. Although we report non-randomized, single-institution data, these results provide reassurance that intensified therapy may not significantly increase the risks of toxicity. Our patient reported outcomes data from a consecutively treated cohort can be useful to guide the expectation of men who are considering post-operative RT with modern radiation techniques.
Data Availability
To ensure compliance with the existing IRB approval letter and HIPAA compliance for patients, the full deidentified dataset cannot be made available without prior written approval from the University of Chicago Hospital IRB. Requests for deidentified data can be made to Dr. Christopher Daugherty, Chairman of the University of Chicago Hospital IRB at cdaugher@medicine.bsd.uchicago.edu.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 2. Cooperberg MR, Broering JM, Carroll PR. Time Trends and Local Variation in Primary Treatment of Localized Prostate Cancer. Journal of Clinical Oncology. 2010;28(7):1117–23. 10.1200/JCO.2009.26.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380(9858):2018–27. 10.1016/S0140-6736(12)61253-7 [DOI] [PubMed] [Google Scholar]
- 4. Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–62. 10.1016/j.juro.2008.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Storkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–30. 10.1200/JCO.2008.18.9563 [DOI] [PubMed] [Google Scholar]
- 6. Cotter SE, Chen MH, Moul JW, Lee WR, Koontz BF, Anscher MS, et al. Salvage radiation in men after prostate-specific antigen failure and the risk of death. Cancer. 2011;117(17):3925–32. 10.1002/cncr.25993 [DOI] [PubMed] [Google Scholar]
- 7. Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. Jama. 2008;299(23):2760–9. 10.1001/jama.299.23.2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moghanaki D, Koontz BF, Karlin JD, Wan W, Mukhopadhay N, Hagan MP, et al. Elective irradiation of pelvic lymph nodes during postprostatectomy salvage radiotherapy. Cancer. 2013;119(1):52–60. 10.1002/cncr.27712 [DOI] [PubMed] [Google Scholar]
- 9. Spiotto MT, Hancock SL, King CR. Radiotherapy after prostatectomy: improved biochemical relapse-free survival with whole pelvic compared with prostate bed only for high-risk patients. Int J Radiat Oncol Biol Phys. 2007;69(1):54–61. [DOI] [PubMed] [Google Scholar]
- 10. Michalski JM, Lawton C, El Naqa I, Ritter M, O'Meara E, Seider MJ, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76(2):361–8. 10.1016/j.ijrobp.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song S, Yenice KM, Kopec M, Liauw SL. Image-guided radiotherapy using surgical clips as fiducial markers after prostatectomy: a report of total setup error, required PTV expansion, and dosimetric implications. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;103(2):270–4. [DOI] [PubMed] [Google Scholar]
- 12. Roach M 3rd, DeSilvio M, Valicenti R, Grignon D, Asbell SO, Lawton C, et al. Whole-pelvis, "mini-pelvis," or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J Radiat Oncol Biol Phys. 2006;66(3):647–53. [DOI] [PubMed] [Google Scholar]
- 13. Alongi F, Fiorino C, Cozzarini C, Broggi S, Perna L, Cattaneo GM, et al. IMRT significantly reduces acute toxicity of whole-pelvis irradiation in patients treated with post-operative adjuvant or salvage radiotherapy after radical prostatectomy. Radiotherapy and Oncology. 2009;93(2):207–12. 10.1016/j.radonc.2009.08.042 [DOI] [PubMed] [Google Scholar]
- 14. Goenka A, Magsanoc JM, Pei X, Schechter M, Kollmeier M, Cox B, et al. Improved Toxicity Profile Following High-Dose Postprostatectomy Salvage Radiation Therapy With Intensity-Modulated Radiation Therapy. European Urology. 2011;60(6):1142–8. 10.1016/j.eururo.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 15. Corbin KS, Kunnavakkam R, Eggener SE, Liauw SL. Intensity modulated radiation therapy after radical prostatectomy: Early results show no decline in urinary continence, gastrointestinal, or sexual quality of life. Practical radiation oncology. 2013;3(2):138–44. 10.1016/j.prro.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 16. Moinpour CM, Hayden KA, Unger JM, Thompson IM, Redman MW, Canby-Hagino ED, et al. Health-Related Quality of Life Results in Pathologic Stage C Prostate Cancer From a Southwest Oncology Group Trial Comparing Radical Prostatectomy Alone With Radical Prostatectomy Plus Radiation Therapy. Journal of Clinical Oncology. 2008;26(1):112–20. 10.1200/JCO.2006.10.4505 [DOI] [PubMed] [Google Scholar]
- 17. Van Cangh PJ, Richard F, Lorge F, Castille Y, Moxhon A, Opsomer R, et al. Adjuvant radiation therapy does not cause urinary incontinence after radical prostatectomy: results of a prospective randomized study. J Urol. 1998;159(1):164–6. [DOI] [PubMed] [Google Scholar]
- 18. Suardi N, Gallina A, Lista G, Gandaglia G, Abdollah F, Capitanio U, et al. Impact of adjuvant radiation therapy on urinary continence recovery after radical prostatectomy. Eur Urol. 2014;65(3):546–51. 10.1016/j.eururo.2013.01.027 [DOI] [PubMed] [Google Scholar]
- 19. Ahlering TE, Eichel L, Edwards R, Skarecky DW. Impact of obesity on clinical outcomes in robotic prostatectomy. Urology. 2005;65(4):740–4. [DOI] [PubMed] [Google Scholar]
- 20. Wolin KY, Luly J, Sutcliffe S, Andriole GL, Kibel AS. Risk of urinary incontinence following prostatectomy: the role of physical activity and obesity. J Urol. 2010;183(2):629–33. 10.1016/j.juro.2009.09.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montgomery JS, Gayed BA, Hollenbeck BK, Daignault S, Sanda MG, Montie JE, et al. Obesity adversely affects health related quality of life before and after radical retropubic prostatectomy. J Urol. 2006;176(1):257–61; discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 22. Jain P, Parsons M. The effects of obesity on the pelvic floor. The Obstetrician & Gynaecologist. 2011;13(3):133–42. [Google Scholar]
- 23. Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: an examination of treatment-related, demographic, and psychosocial factors. Cancer. 2001;92(6):1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. The New England journal of medicine. 2008;358(12):1250–61. 10.1056/NEJMoa074311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
To ensure compliance with the existing IRB approval letter and HIPAA compliance for patients, the full deidentified dataset cannot be made available without prior written approval from the University of Chicago Hospital IRB. Requests for deidentified data can be made to Dr. Christopher Daugherty, Chairman of the University of Chicago Hospital IRB at cdaugher@medicine.bsd.uchicago.edu.