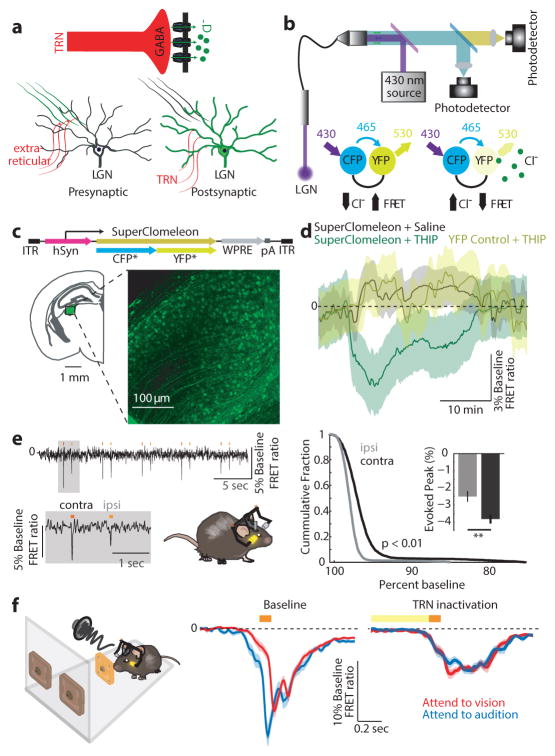
Figure 5. Measuring bulk intracellular [Cl−] in vivo shows dynamic changes in LGN inhibition during behavior.
(a) Possible mechanisms for LGN firing rate modulation; extra-reticular inputs can change activity by presynaptic inhibition of feedforward excitation while visTRN inhibits LGN directly. (b) FRET photometry setup and schematic of CFP-to-YFP FRET. (c) Cloning of the FRET-based Cl− indicator superclomeleon into an AAV followed by in vivo expression in the LGN. (d) Pharmacological confirmation of the technical feasibility of superclomeleon FRET for GABAa mediated increase in intracellular [Cl−] by injection of the GABAa agonist THIP. Note that the YFP control mice did not show similar signals (n = 3 mice per condition, shaded errors are 95% confidence intervals). (e) Mice showed visual-evoked superclomeleon FRET responses that are stronger for the contralateral eye, as would be predicted (n = 3 mice, p < 0.05, Wilcoxon Rank-sum Test). Yellow bars mark the display of the light stimuli. (f) Left Cartoon depiction of photometry in the cross-modal task, where the visual stimulus was signaled through a head-mounted LED as in Fig. 4 (see Supplementary Video 1 for illustration). Middle Differential visual-evoked [Cl−] LGN responses in relation to the modality anticipated (363 visual and 274 auditory correct trials from 6 mice). Shaded errors are 95% confidence intervals. Note that ‘attend to audition’ trials showed an earlier increase in [Cl−] (decreased superclomeleon FRET) and the separation between the two traces started prior to stimulus onset, consistent with differential anticipatory changes of visTRN activity. Right Optogenetic TRN inactivation eliminates this differential response (101 visual and 82 auditory correct trials from 3 mice).