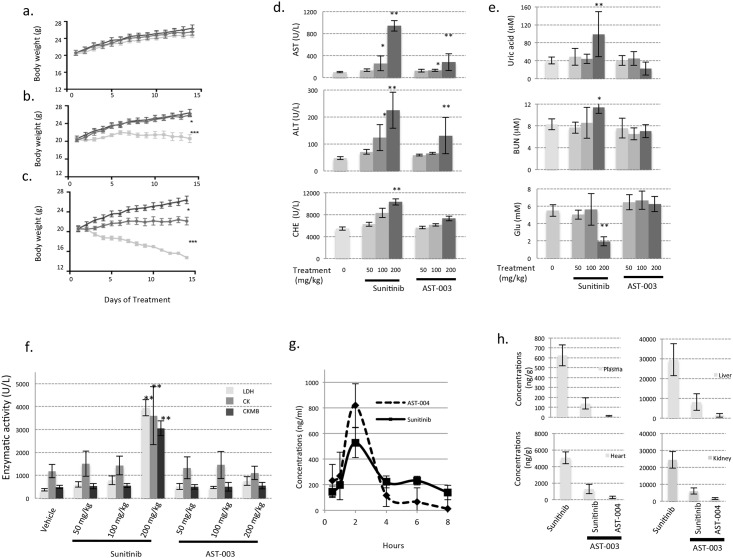
Fig 3. AST-003 is better tolerated in mice.
The MTD experiments were performed as described in the Methods and Materials. AST-003 or Sunitinib was orally administered once daily at a dose of 50 mg/kg (a), 100 mg/kg (b), and 200 mg/kg (c). The body weights of mice treated with AST-003 (circle), Sunitinib (square) and vehicle (triangle) were measured. All data are the means of 5 mice, with error bars representing the standard deviation. (Statistical analysis: (b) AST-003 vs. Sunitinib, *** P<0.001; Sunitinib vs. Vehicle, *** P<0.001; (c) AST-003 vs. Vehicle, * P<0.05; AST-003 vs. Sunitinib, *** P<0.001; Sunitinib vs. Vehicle, *** P<0.001) At the end of the MTD assays, the biochemical activities of AST, ALT, and CHE (d), Glucose(Glu), Urea acid, blood urea nitrogen (BUN) (e), LDH, CK, and CKMB (f) were measured (* P<0.05; ** P<0.01). (g) AST-003 was orally administered at a dose of 40 mg/kg. The concentrations of AST-004 and Sunitinib were then measured. All data are the means of replicates from 5 mice, with error bars representing the standard deviation. (h) AST-003 and Sunitinib were orally administered at a dose of 40 mg/kg. The concentrations of AST-004 and Sunitinib were then measured 8 h after administration. All data are the means of replicates from 5 mice, with error bars representing the standard deviation.