Abstract
Objective
To investigate the association between Chlamydia trachomatis (CT) infection seropositivity and gastroschisis.
Study Design
In this case-control study we enrolled pregnant women either prenatally diagnosed with gastroschisis (cases, n=33) or with a normal ultrasound (controls, n=66). Both groups attended the University of Utah's Maternal Fetal Medicine Diagnostic Center for their diagnostic ultrasound or because of a community obstetrician referral. Participants completed a structured interview on potential risk factors. Anti-CT IgG1 and IgG3 were measured by a CT elementary body ELISA.
Result
Median age at sexual debut was lower and reported sexual partner number higher in cases compared to controls. Risk factors for gastroschisis included having ≥3 sexual partners (OR=3.3, 95% CI 1.2, 9.4), change in partner from the previous pregnancy (OR=3.6, 95% CI 0.9, 13.9), and anti-CT IgG3 seropositivity (age-adjusted OR=3.9, 95% CI: 1.1, 13.2).
Conclusion
Anti-CT IgG3 seropositivity was associated with greater than a 3-fold risk for gastroschisis.
Introduction
The increasing prevalence of gastroschisis, a congenital defect of the abdominal wall, has been documented over the past several decades in the U.S.1 and around the world.2-6 In the U.S., the greatest increase is observed among young women, especially those less than 20 years of age.1 The cause for this increase and for the dramatic difference by maternal age are unknown. The rapidity of the increase suggests that environmental factors play a role. A study from the National Birth Defects Prevention Study reported that women delivering an infant with gastroschisis were four times more likely to report a genitourinary (GU) infection (i.e., urinary and sexually transmitted infections [STIs]) during the periconceptional period than control mothers.7 That study also suggested a specific role of Chlamydia trachomatis (CT) infection, which accounted for 43% of GU reported infections among case mothers compared to 18% among controls. GU infections were also disproportionately more frequent in women <25 years of age. Two additional studies reported an association between periconceptional GU infection and risk for gastroschisis.8,9 Several factors suggest that CT infection should be further investigated as a potential risk factor for gastroschisis: 1) it is the most prevalent sexually transmitted bacterial infection; 2) prevalence is highest in women aged 15-24 years, and remains high despite a national chlamydia control program;10 and 3) the rising rate of CT infection among young women has paralleled the increasing prevalence of gastroschisis.
However, investigating CT infection as a risk factor for gastroschisis is challenging, for several reasons: 1) subclinical CT infections are frequent (up to 75% of CT-infected women are asymptomatic11) which makes using maternal self-report as a marker for CT exposure less ideal due to the potential exposure misclassification, and 2) CT infections may resolve spontaneously (i.e., without treatment) and therefore a negative CT test (i.e., nucleic acid amplification test – NAAT) at a prenatal visit does not rule out a prior periconceptional CT exposure.12 Hence, more objective and sensitive approaches are needed to evaluate CT exposure. One such proposed measure is a CT-specific antibody response. Geisler et al.13 recently reported that IgG1 and IgG3, detected by a CT elementary body (EB) ELISA, comprised the predominant anti-CT serum antibody response, and the seropositivity rate detected by CT EB ELISA was higher than a commercial CT ELISA (Medac). Our primary objective was to evaluate the association between gastroschisis and CT seropositivity, which was performed using the CT EB ELISA. Secondary objectives included: 1) assess study participation rates of pregnant women prenatally diagnosed with and without gastroschisis; 2) determine the utility of medical record review in documenting prenatal STI screening; and 3) evaluate associations of sexual history with gastroschisis.
Methods
Participant Recruitment
Because of the rarity of gastroschisis, we used a case-control design to address the study objectives. Pregnant women were recruited from the Maternal-Fetal Diagnostic Center (MFDC) at the University of Utah hospital either at the time of their routine diagnostic ultrasound (i.e., 18-20 weeks gestation) or referred from the University's obstetric clinics, the Teen Mother Child Program, and community obstetricians and midwives. For some case women, an ultrasound confirmation of gastroschisis occurred later in the second trimester, but the reason was not known. Study coordinators from the Obstetrics and Gynecology Research Network (OGRN), trained to sensitively approach women with abnormal ultrasound findings, identified potentially eligible pregnant women prenatally diagnosed with gastroschisis (cases) and approached them during their visit to the MFDC to provide information about the study. Those who desired to participate provided written informed consent.
For each enrolled case, two eligible control women attending the MFDC for any reason with a normal ultrasound were matched by gestational age (+/- one week), recruited and consented. Approximately 40% of babies born with gastroschisis are delivered to women <20 years of age.1 This study did not restrict participation by maternal age. For unmarried women ≤17 years of age, parental consent was required for participation. Women were not eligible if they did not speak or understand English or Spanish. The study was approved by the Institutional Review Board of the University of Utah.
Data Collection and Testing
After obtaining consent, the study coordinator administered a questionnaire in English or Spanish that focused on demographics, previous pregnancies and change in partners, selected exposures (infections, fevers, tobacco use, medications, and illicit drug use), and sexual history. Questions about previous pregnancy history, current pregnancy information, and maternal exposures were taken from the National Birth Defect Prevention Study's questionnaire (used since 1997) and questions related to sexual history and behavior were taken from the validated National Health and Nutrition Examination Survey. Women were also asked to sign a release for their prenatal medical records, which were reviewed to determine if screening for STIs was performed at the first prenatal visit.
The study coordinator obtained blood from participants after completion of the questionnaire. Each participant's interview and samples were assigned a unique study ID number. Urine was tested on the first 33 enrollees at the Utah State Laboratory at the Utah Department of Health for evidence of active CT and/or gonococcal infection using the highly sensitive NAAT (APTIMA Combo 2 assay (Gen-Probe, San Diego, CA).14 The urine NAAT screen was discontinued since we were not able to detect active CT infections at the time of enrollment (third trimester) among the first 33% participants. This suggests that subclinical periconceptional CT infections either cleared or if clinically diagnosed, were treated with antibiotics. Serum was extracted from blood and stored in -80° freezers until testing. Participants were also asked to provide written consent and permission for blood DNA storage for future genetic studies.
Anti-CT Antibody Detection
At first, we measured serum anti-CT IgG antibodies using the commercially available SeroELISA Chlamydia IgG kit (Savyon Diagnostics, Israel) per the manufacturer's recommendations. However, after testing the initial 16 cases and 28 controls, only one case was IgG seropositive, one case had equivocal results, and no controls were seropositive. The sensitivity of this assay was concerning. We switched to the CT EB ELISA, an assay in which Geisler et al.13 had reported detection of >15% more CT infections than the commercially available Medac anti-CT IgG antibody assay. These 44 samples were retested with the additional samples (n=55) using the methodology described below. Laboratory testing was conducted without knowledge of case or control status.
Formalin-fixed, density gradient purified EBs of CT serovars D, F and J obtained from the University of Washington were used as antigens in the EB-ELISA. Working in collaboration with Dr. Geisler from the University of Alabama at Birmingham, we established an EB ELISA using methodology similar to those he published.13 Briefly, density gradient purified EBs were fixed overnight at 4°C in 10 mM phosphate-buffered saline (PBS) containing 0.2% formalin. After fixation, EBs were washed once with PBS and re-suspended in PBS with 0.02% formalin. Equal volumes of each serovar (2 mg/mL) were combined to make a mixture of serovars D, F, and J and used to coat 96–well Immunlon 2 HB U-bottoms plates (Thermo Scientific, 2 μg EB/well). Detection of CT-specific antibodies in sera was performed with alkaline phosphatase–labeled anti-human IgG monoclonal antibodies (anti-IgG1 [a pool of clones 4E3, Southern Biotech; and HP6069; Cal Biochem], anti-IgG3 [clone HP6050; Southern Biotech]) and Sigma Fast p-nitrophenyl phosphate substrate (1 mg/mL; Sigma). The optical density (OD) of the reactions was measured at 405 nm using SPECTRAmax PLUS 384 plate reader and analyzed with SoftMax® Pro 4.8 software. To establish cutoff values for positive IgG1 and IgG3 responses, sera were collected from 10 low-risk volunteers (negative controls) and samples from five women with confirmed active genital CT infection detected in urine or cervical swab by NAAT (positive controls). Samples were diluted 1:32 in ELISA buffer (2% BSA, 0.05 M Tris base, 0.15 M NaCl, pH=7.2-7.5) and plated in triplicates onto blocked EB-coated plates. After incubation, plates were extensively washed and probed with either anti-CT IgG1 or IgG3 monoclonal antibodies. Mean OD405 readings for low-risk samples and 3 standard deviations were considered as the cutoff point for positive responses (seropositivity: IgG1≥0.1, IgG3≥0.08). OD405 readings for all positive control samples were far above IgG1 and IgG3 cutoff values. A positive control sample and negative control sample were used as internal controls in all plates on which participant sera were tested. The reported serologic responses of all participants represent the mean of triplicate determinations of 1:32 diluted serum for each IgG subclass response.
Statistical Analyses
Descriptive statistics were used to compare frequencies of self-reported data. Associations of participant characteristics and seropositivity with gastroschisis were evaluated with the Chi-squared test or Fisher's exact test (cell size <5) and nonparametric tests as appropriate (median test, Wilcoxon Rank Sum test). Crude (cOR) and maternal-age adjusted odds ratios (aOR) with 95% confidence intervals (CI) were calculated using conditional logistic regression and stratified analyses to evaluate maternal age stratum specific estimates. Analyses were conducted in SAS 9.1 (SAS Institute, Cary, NC, 2002-3).
Results
Of 113 eligible pregnant women (34 cases; 81 controls) 99 (86%) participated:33 (97.1%) cases and 66 (81.5%) controls. Gestational age at enrollment was on average 27.3 weeks (range 16-37 weeks) for cases and 27.7 weeks (range 18-38.2 weeks) for controls.
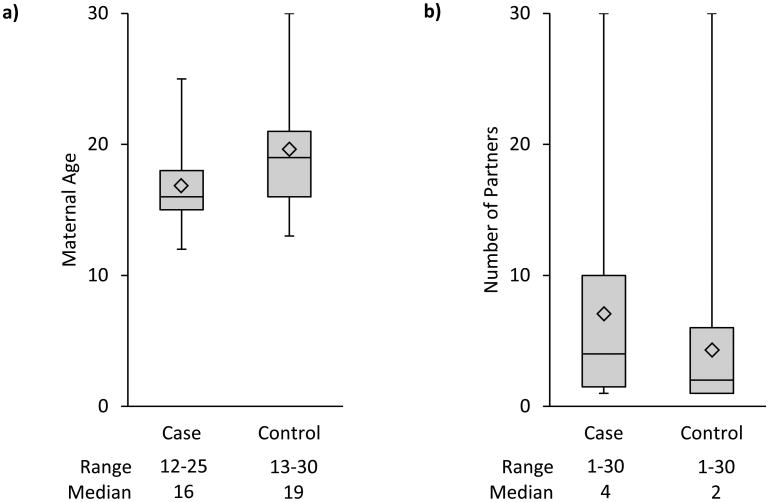
Cases were significantly more likely to be younger, less educated, smoke cigarettes, report a previous STI, and among those with a previous pregnancy, report a different partner with their current pregnancy than the most recent pregnancy (Table 1). Compared to controls, cases reported a significantly lower median age at sexual debut (defined as first reported age at vaginal intercourse) (p=0.0002) and a greater number of sexual partners since their debut (p=0.058) (Figure 1a, 1b). Both the number of sexual partners since becoming sexually active (sexual debut) and a change in partner from the previous pregnancy were associated with an increased risk for gastroschisis (Table 2). Among women <25 years of age, cases reported more sexual partners than controls (p=0.006) compared to women ≥25 (p=.66). When specifically queried about previous STI diagnoses, cases more often reported a prior CT infection (5 [15.2%] vs. 1 [1.5%]), genital herpes (1 [3.0%] vs. 1 [1.5%]), and gonorrhea (1 [3%] vs. 0%) (any self-reported STI: aOR=6.7, 95%CI: 0.7, 58.8). Among women reporting illicit drug use during the periconceptional period, only marijuana use was reported.
Table 1. Characteristics of participating case and control women.
| Characteristic | Cases n=33 (%) | Controls n=66 (%) | P-valuea | ||
|---|---|---|---|---|---|
| Maternal age (years) | |||||
| <20 | 9 | 27.2 | 5 | 7.6 | 0.002 |
| 20-24 | 17 | 51.5 | 24 | 36.4 | |
| 25-29 | 6 | 18.2 | 21 | 31.8 | |
| ≥30 | 1 | 3.0 | 16 | 24.2 | |
| Race/Ethnicity | |||||
| White, non-Hispanic | 30 | 90.9 | 52 | 78.8 | 0.59 |
| Hispanic | 2 | 6.1 | 8 | 12.1 | |
| Other | 1 | 3.0 | 6 | 9.1 | |
| Education (years) | |||||
| < 12 | 6 | 18.2 | 3 | 4.5 | 0.04 |
| 12 | 9 | 27.2 | 13 | 19.7 | |
| > 12 | 18 | 54.5 | 50 | 75.8 | |
| Preconception BMIb | |||||
| <18.5 | 3 | 9.1 | 2 | 3.0 | 0.25 |
| 18.5-24.9 | 20 | 60.6 | 38 | 57.6 | |
| 25.0-29.9 | 8 | 24.2 | 17 | 25.8 | |
| ≥30.0 | 1 | 3.0 | 9 | 13.6 | |
| Cigarette smokingc | |||||
| No | 21 | 63.6 | 60 | 90.9 | 0.0009 |
| Yes | 12 | 36.1 | 6 | 9.1 | |
| Second hand smoke | |||||
| No | 25 | 75.8 | 59 | 89.3 | 0.07 |
| Yes | 8 | 24.2 | 7 | 10.6 | |
| Illicit drugs | |||||
| No | 28 | 84.8 | 59 | 89.3 | 0.51 |
| Yes | 5 | 15.2 | 7 | 10.6 | |
| First pregnancy | |||||
| No | 16 | 48.5 | 38 | 57.6 | 0.39 |
| Yes | 17 | 51.5 | 28 | 42.4 | |
| Gravidity | |||||
| 0 | 17 | 51.5 | 29 | 43.9 | 0.21 |
| 1 | 9 | 27.2 | 15 | 22.7 | |
| 2 | 6 | 18.2 | 10 | 15.2 | |
| ≥3 | 1 | 3.0 | 12 | 18.2 | |
| History of STId | |||||
| No | 28 | 84.8 | 65 | 98.5 | 0.015 |
| Yes | 5 | 15.2 | 1 | 1.5 | |
| Number of sexual partnerse | |||||
| 1 | 8 | 24.2 | 32 | 48.5 | 0.07 |
| 2 | 3 | 9.1 | 6 | 9.1 | |
| ≥3 | 21 | 63.6 | 28 | 42.4 | |
| Same partner from previous pregnancy | |||||
| No | 10 | 62.5 | 5 | 13.5 | 0.0005 |
| Yes | 6 | 37.5 | 32 | 86.5 | |
P values were calculated using Chi squared test or if cell size was <5 Fisher's exact test
BMI=body mass index in kg/m2
Cigarette smoking one month before to one month after conception
STI=sexually transmitted infection
One case – did not report number of sexual partners
Figure 1.
a: Maternal age at sexual debut among cases (n=33) and controls (n=66)
b: Number of sexual partners by case (n=32) and control (n=66) status
Table 2. Association for sexual history of case and control women.
| Number of sexual partnersa | Case | Control | cOR (95%CI) |
|---|---|---|---|
|
|
|||
| 1 | 8 | 32 | Ref |
| 2 | 3 | 6 | 2.3 (0.5, 12.0) |
| ≥3 | 21 | 28 | 3.3 (1.2, 9.4) |
| Previous pregnancy with same partner | |||
| Yes | 6 | 32 | ref |
| No | 10 | 5 | 3.6 (0.9, 13.9) |
One case – did not report number of sexual partners
Abbreviations: cOR, crude odds ratio; 95%CI, 95% confidence interval
All participating cases and controls consented to have their medical records reviewed. Evidence of a negative screening NAAT for chlamydia and gonorrhea at the first prenatal visit was documented in the prenatal medical records for 19 (57.6%) cases and 37 (56%) controls. The remaining medical records either did not document that a screening NAAT was performed (10 [30.3%] cases; 28 [42.4%] controls), or if performed, did not document the results (4 [12.1%] cases; 1 [1.5%] controls). No medical records included the screening method used to determine a negative or positive screen.
Anti-CT Antibody Detection by CT EB- ELISA
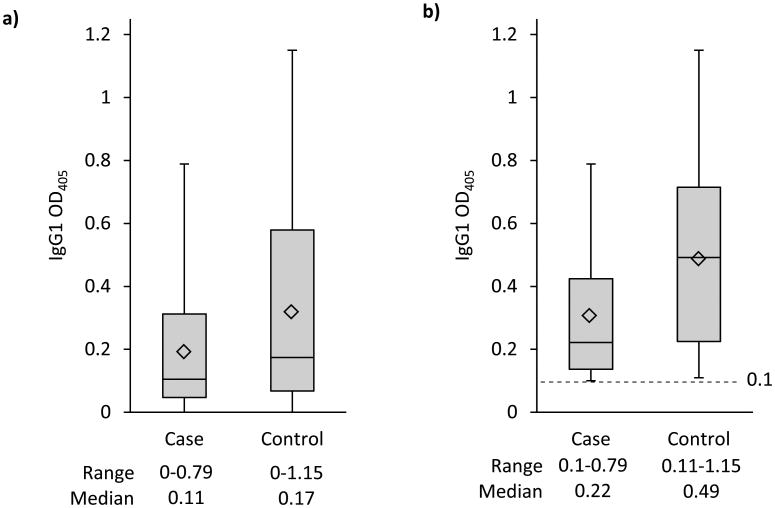
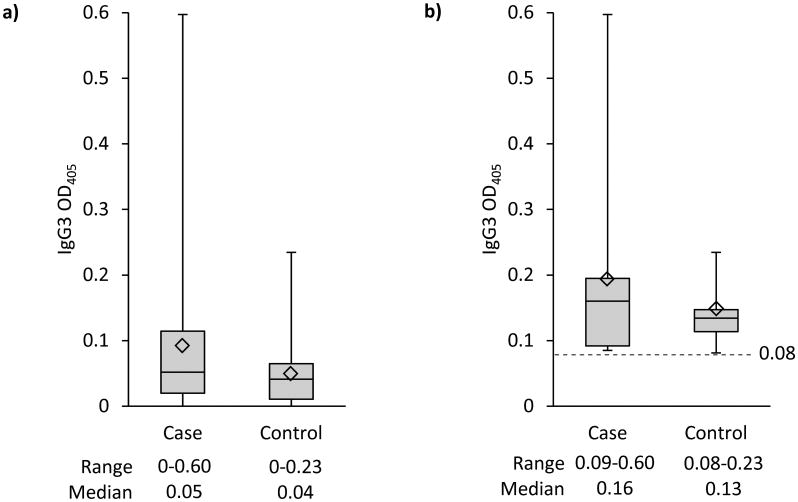
Overall, 62 (62.6%) participants were CT seropositive by IgG1 and 25 (25.3%) by IgG3; 15 (15.2%) were CT positive by both IgG1 and IgG3. Anti-CT IgG1 seropositivity was not significantly associated with gastroschisis (Table 3). Anti-CT IgG3 seropositivity (39.4% of cases and 18.2% of controls) was associated with greater than a three-fold increased risk for gastroschisis (cOR=3.4, 95%CI: 1.2, 9.8; aOR=3.9, 95%CI: 1.1, 13.2) (Table 3). No association was observed for IgG1 seropositivity among women <25 (OR=0.8, 95%CI 0.28, 2.5; p=0.84) or ≥25 years of age (OR=0.8, 95%CI 0.2, 4.2; p=0.81). Whereas, the risk of gastroschisis for women <25 years of age was increased among those with IgG3 seropositivity (cOR=3.9, 95%CI 1.0, 14.6; p=0.02) compared to women ≥25 years of age (cOR=2.7, 95%CI 0.5, 14.7; p=0.24) (data not shown). The magnitude of the anti-CT IgG1 response detected was lower in cases than controls (median OD, 0.11 vs. 0.17; p=0.118) (Figure 2a), and among participants IgG1 seropositive, the magnitude of the response was lower in cases compared to controls (median OD 0.22 vs. 0.49; p=0.013) (Figures 2b). In contrast, the magnitude of the anti-CT IgG3 response detected did not differ between cases and controls (median OD 0.05 vs. 0.04; p=0.135) (Figure 3a),and among those who were IgG3 seropositive the magnitude of response also did not significantly differ in cases compared to controls (median OD, 0.16 vs. 0.13; p=0.73) (Figure 3b).
Table 3. Association for anti-chlamydia specific antibodies and gastroschisis.
| Anti-Chlamydia IgG1 Seropositive | Anti-Chlamydia IgG3 Seropositive | |||
|---|---|---|---|---|
|
|
|
|||
| Yes n (%) | No n (%) | Yes n (%) | No n (%) | |
|
| ||||
| Cases | 20 (60.6%) | 13 (39.4%) | 13 (39.4%) | 20 (60.5%) |
| Controls | 42 (63.6%) | 24 (36.4%) | 12 (18.2%) | 54 (81.8%) |
|
|
||||
| cOR = 0.9 (95% CI: 0.4, 2.0) aOR = 0.8 (95% CI: 0.3, 2.0) |
cOR = 3.4 (95%CI: 1.2, 9.8) aOR = 3.9 (95% CI: 1.1, 13.2) |
|||
Abbreviations:
cOR, crude odds ratio; 95%CI, 95% confidence interval
aOR, odds ratio, adjusted for maternal age
Figure 2.
a: Anti-Chlamydia trachomatis IgG1 OD405 reading by case (n=33) and control (n=66) status
b: Seropositive (≥ 0.1 OD405) anti-Chlamydia trachomatis IgG1 by case (n=20) and control (n=42) status
Figure 3.
a: Anti-Chlamydia trachomatis IgG3 OD405 reading by case (n=33) and control (n=66) status
b: Seropositive (≥ 0.08 OD405) anti-Chlamydia trachomatis IgG3 by case (n=13) and control (n=12) status
Discussion
The findings show that maternal anti-CT IgG3 seropositivity was associated with a 3.9-fold increased risk for gastroschisis in the offspring. A study by Geisler et al.13 demonstrated that anti-CT IgG1 and IgG3 comprised the predominant anti-CT serum antibody responses. It is believed that IgG3 is involved early in response to infection and then is short-lived with IgG1 emerging as the major effector in eradicating infection.15 Geisler et al.13 reported some decline in the magnitude of the IgG3 response within 6 months of chlamydia treatment. It is plausible that detection of anti-CT IgG3 in this cohort of pregnant women suggests a more recent CT exposure, potentially in the periconceptional period where CT infection could contribute to the development of gastroschisis.
The mechanism by which CT infection could lead to gastroschisis is not known. One mechanism might be proinflammatory cytokines induced by chlamydia impairing normal abdominal wall development. CT infections can lead to salpingitis, infertility, ectopic pregnancy, reactive arthritis, and perihepatitis.16 The innate and adaptive immune response induces systemic inflammation leading to cytokine production which has the potential to damage cells.
The sexual history of participants was consistent with behaviors that are known to increase the likelihood of acquiring an STI.11,17,18 Compared to controls, cases as a group were sexually active at a younger age, had more sexual partners, and more frequently reported a change in partner from their previous pregnancy. Sexually active adolescents and young women are much more susceptible to sexually transmitted infections. Unlike the squamous cells in the cervical epithelium of adult females, the genital tracts of adolescent and young women are lined by immature undifferentiated columnar epithelial cells.17 This immature epithelium is thought to be more susceptible to pathogens and is the preferred site for C. trachomatis and Neisseria gonorrhea.17 In epidemiologic studies, young maternal age has been the strongest and most consistent risk factor for gastroschisis.1 The mechanism underlying this association is not understood but could be related to the combination of exposure (increased risk for sexually transmitted infections due to more risky behaviors) and susceptibility (the biology of the reproductive tract). Eight (24.2%) cases and 19 (28.8%) controls did not have any evidence (no IgG1 or IgG3 seropositivity) of prior CT infection, suggesting that other infections or environmental exposures are responsible for gastroschisis.
The study had several strengths. Participation in the study for both case and control women was high, demonstrating that women are willing to participate in a research study during pregnancy despite their new diagnosis of a fetal anomaly. We used a highly sensitive serologic test, CT EB ELISA, to maximize the detection of a prior known or subclinical CT infection; our study also confirmed that CT EB ELISA detects chlamydia antibodies more often than commercially available assays. The use of a sensitive test is crucial as it helps reduce (or eliminate) the potential for exposure misclassification which may influence the accuracy of detected associations between CT infection and risk for gastroschisis. This study was the first step to determine whether CT seropositivity increases the risk for gastroschisis. Whether it is the pathogen or the inflammatory response as a result of the pathogen requires further research. We also found that more than 40% of prenatal medical records lacked detailed information on whether screening for STIs was conducted during the first prenatal visit or if conducted, the results were not listed. There is also the possibility that some of the negative screens were false negatives since the immune system can clear a subclinical local chlamydia infection.11
The findings from this study must be interpreted in the context of its limitations. An important limitation is that this was an exploratory pilot study to investigate the feasibility for the methodology with a small sample of eligible cases. The focus on prenatally diagnosed cases meant that we would have missed a woman whose affected pregnancy was not prenatally diagnosed. Among Utah residents, approximately 90% of cases are prenatally diagnosed and referred to the University of Utah for delivery. Only a small number of women (n=3 or 10%) would not have had the opportunity to participate in the study because they were not prenatally diagnosed with gastroschisis. The gestational age at enrollment was later than anticipated. We estimated initially that the majority of the diagnoses and enrollment would have occurred at 18 to 20 weeks gestation. In reality, the average gestational-age at enrollment was 27 weeks, which may have influenced our ability to detect active chlamydia infection by NAAT. Community obstetric providers may have referred their patients for a MFDC confirmation ultrasound weeks after the initial ultrasound diagnosing a gastroschisis. Another limitation of the study was that we were not able to test for other pathogens known to cause STIs. However, serum samples were banked for future studies on additional viral or bacterial pathogens. Finally, the questionnaire was administered during pregnancy, soon after the prenatal diagnosis of a fetal abnormality (gastroschisis) and 76% of the cases were less than 25 years of age compared to 44% of controls. Young women may feel more comfortable, in general, answering questions about their sexual encounters, potentially inflating the findings on sexual history.
GU infections, and CT infections specifically, could represent an intermediate step between risky sexual behaviors and increased risk for gastroschisis: these behaviors increase the likelihood to acquire one or more sexually transmitted infections, which in turn may be particularly harmful in pregnancies of young women because of the particular susceptibility of their reproductive system. Finding an increased risk for gastroschisis among women with evidence of a recent CT infection is the first step in understanding the association with this pathogen and the host's subsequent systemic inflammatory response. CT has the ability to induce a systemic inflammatory response, the magnitude of which may differ in cases compared to controls as a result of the individual's immunogenetic determinants. Future research should include evaluation of the influence of CT-specific cellular immune responses on gastroschisis risk. In addition, this study underscores the important role of biomarker-based studies in birth defect epidemiology. Because the majority of CT infections in women are subclinical, association can be easily missed or distorted if appropriate biomarkers such as serum antibodies are not used, and associations are exclusively based on self-reports of such challenging exposures.
Conclusion
Anti-CT IgG3 seropositivity was associated with more than a 3-fold risk for gastroschisis, which in combination with the association of risky sexual behavior suggests recent CT infection around or shortly after conception increases gastroschisis risk.
Acknowledgments
This study was supported by a Cooperative Agreement (Number U01DD000490) from the Centers for Disease Control and Prevention to the Utah Center for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Kirby RS, Marshall J, Tanner JP, Salemi JL, Feldkamp ML, Marengo L, et al. Prevalence and correlates of gastroschisis in 15 states, 1995-2005. Obstet Gynecol. 2013;122(2 Pt 1):275–281. doi: 10.1097/AOG.0b013e31829cbbb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hougland KT, Hanna AM, Meyers R, Null D. Increasing prevalence of gastroschisis in Utah. J Pediatr Surg. 2005;40:535–540. doi: 10.1016/j.jpedsurg.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Laughon M, Meyer R, Bose C, Wall A, Otero E, Heerens A, Clark R. Rising birth prevalence of gastroschisis. J Perinatol. 2003;23:291–293. doi: 10.1038/sj.jp.7210896. [DOI] [PubMed] [Google Scholar]
- 4.Collins SR, Griffin MR, Arbogast PG, Walsh WF, Rush MR, Carter BS, et al. The rising prevalence of gastroschisis and omphalocele in Tennessee. J Pediatr Surg. 2007;42:1221–1224. doi: 10.1016/j.jpedsurg.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Vu LT, Nobuhara KK, Laurent C, Shaw GM. Increasing prevalence of gastroschisis: population-based study in California. J Pediatr. 2008;152:807–811. doi: 10.1016/j.jpeds.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Castilla EE, Mastroiacovo P, Orioli IM. Gastroschisis: international epidemiology and public health perspectives. Am J Med Gen Sem Med Gen. 2008;148C:162–179. doi: 10.1002/ajmg.c.30181. [DOI] [PubMed] [Google Scholar]
- 7.Feldkamp ML, Reefhuis J, Kucik J, Krikov S, Wilson A, Moore CA, et al. Maternal genitourinary infections and the risk of gastroschisis – national birth defects prevention study, 1997-2003. Br Med J. 2008;336:1420–1423. doi: 10.1136/bmj.39567.509074.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draper ES, Rankin J, Tonks AM, Abrams KR, Field DJ, Clarke M, Kurincsuk JJ. Recreational drug use: a major risk factor for gastroschisis. Am J Epidemiol. 2008;167:485–491. doi: 10.1093/aje/kwm335. [DOI] [PubMed] [Google Scholar]
- 9.Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518–525. doi: 10.1093/aje/kwu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2013. Atlanta: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 11.Geisler WM, Stamm WE. Current Diagnosis and treatment of sexually transmitted diseases. McGraw Hill Medical; New York: 2007. Gential Chlamydia infections; pp. 75–83. [Google Scholar]
- 12.Geisler WM. Duration of untreated uncomplicated genital Chlamydia trachomatis infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis. 2010;201:S104–S113. doi: 10.1086/652402. [DOI] [PubMed] [Google Scholar]
- 13.Geisler WM, Morrison SG, Doemland ML, Igbal, Su J, Mancevski A, Hook EW, III, Morrison RP. Immunoglobulin-specific responses to Chlamydia elementary bodies in individuals with and at risk for genital chlamydial infection. J Infect Dis. 2012;206:1836–1843. doi: 10.1093/infdis/jis621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schachter J, Hook EW, Martin DH, Willis D, Fine P, Fuller D, Jordan J, Janda WM, Chernesky M. Confirming positive results of nucleic acid amplification tests (NAATs) for Chlamydia trachomatis: all NAATs are not created equal. J Clin Microbiol. 2005;43:1372–1373. doi: 10.1128/JCM.43.3.1372-1373.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins AM, Jackson KJL. A temporal model of human IgE and IgG antibody function. Front Immunol. 2013;4(235):1–6. doi: 10.3389/fimmu.2013.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peipert JF. Clinical practice. Genital chlamydial infections. N Engl J Med. 2003;349(25):2424–2430. doi: 10.1056/NEJMcp030542. [DOI] [PubMed] [Google Scholar]
- 17.Arrington-Sanders R, Dyson J, Ellen J. Sexually transmitted diseases in adolescents. In: Klausner JD, Hook EW III, editors. Current diagnosis and treatment of sexually transmitted diseases. McGraw Hill Medical; New York: 2007. pp. 160–166. [Google Scholar]
- 18.Forhan SE, Gottlieb SL, Sternberg MR, Xu F, Datta SD, McQuillan GM, et al. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics. 2009;124:1505–1512. doi: 10.1542/peds.2009-0674. [DOI] [PubMed] [Google Scholar]