Figure 1. Molecular pathways for reduced B cell responses in aging.
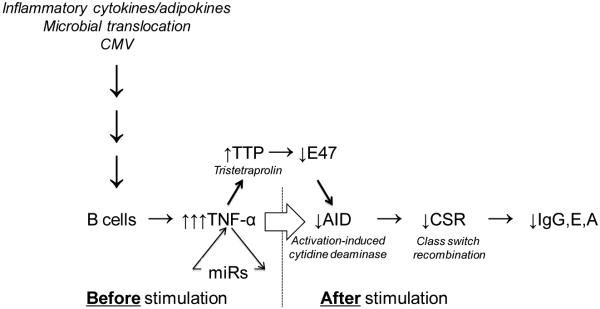
The production of high-affinity, class-switched antibodies (IgG/E/A) is decreased both in vivo and in vitro as a consequence of reduced class switch recombination (CSR) with aging. CSR is a DNA recombination event regulated by activation-induced cytidine deaminase (AID), the enzyme responsible for opening the double stands of DNA in the S (switch) regions of Immunoglobulin genes. AID also regulates somatic hypermutation and therefore is crucial for the production of protective antibodies. AID is decreased in stimulated B cells from aged mice and humans and is transcriptionally regulated by E47, the Helix-Loop-Helix transcription factor, which is decreased in aged B cells by mRNA stability. E47 mRNA stability is down-regulated by tristetraprolin (TTP), a negative regulator of the stability of transcription factor and cytokine mRNAs. TTP is higher in unstimulated B cells from aged mice and humans, and is positively regulated by B cell intrinsic TNF-α and particular inflamma-micro-RNAs (miRs), such as miR-155 and miR-16. Levels of TTP, TNF-α and miRs in unstimulated B cells are positively regulated by serum levels of pro-inflammatory cytokines and adipokines, microbial translocation and persistent infections with viruses such as CMV. We have shown that the age-related increase in these markers is associated with TNF-α production by unstimulated B cells and that this “pre-activated” phenotype of the B cells renders them incapable of being optimally stimulated by exogenous antigens, mitogens or vaccines.
