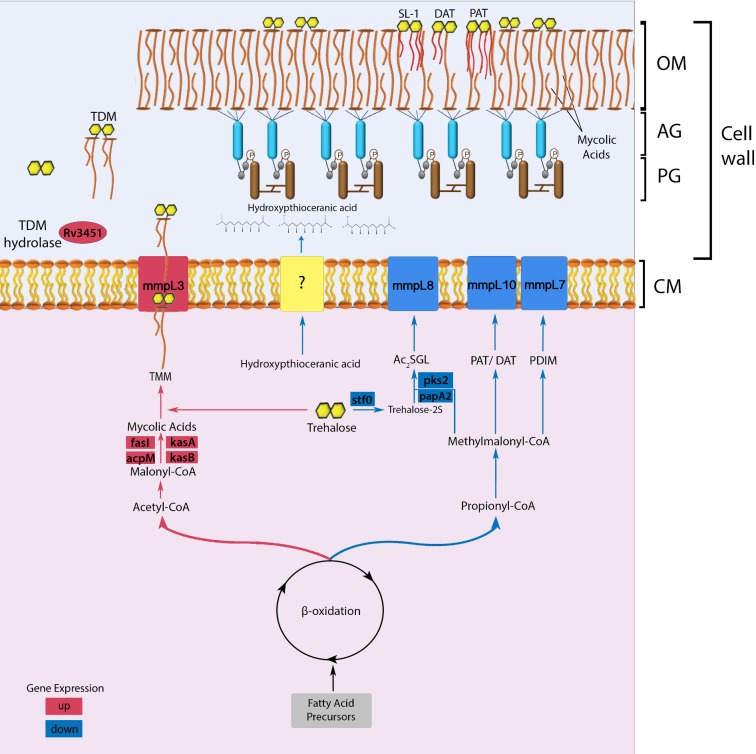
Figure 5.
Proposed model of cell wall lipid reorganization in M. tuberculosis. The beta-oxidation cycle of M. tuberculosis has two pathways that lead to the synthesis of either acetyl-CoA/malonyl CoA or propionyl-CoA/methylmalonyl-CoA (Munoz-Elias and McKinney 2005). The synthesis of these metabolites is determined according to cytoplasmic availability of fatty acid precursors. In wild-type M. tuberculosis, the mce1 operon (a putative MA importer) is repressed during the first 4–8 weeks of infection in mice (Uchida et al. 2007), or when the organism is intracellular (Casali, White and Riley 2006). During this period, the acetyl-CoA pathway may be favored, which causes gradual free mycolate (FM) accumulation on the bacterial surface (Cantrell et al. 2013) with a block in MA import (indicated in red) (Dunphy et al. 2010), while diacylated sulfoglycolipid (Ac2SGL), poly and diacyltrehalose (PAT/DAT) and phthiocerol dimycocerosates (PDIM) export decreases (indicated in blue). The cell wall thickened with excess FM allows M. tuberculosis to resist host effector cells and molecules. However, the thickened cell wall also limits nutrient uptake, which eventually triggers a starvation response, which will induce the mce1 operon to be turned on and M. tuberculosis to synthesize the MA transporter in the inner membrane to import MA from its surface back into the cytoplasm (Dunphy et al. 2010). The imported MA degraded in the cytoplasm enters the propionyl CoA/methylmalonyl CoA synthetic pathway that will favor transport of proinflammatory lipids to the bacterial surface and bacterial replication. Although we have no evidence that these lipid changes actually occur in vivo, we hypothesize that these lipid changes in the cell wall may induce different host responses that determine clinical outcome of an infection. The rapid progression to death in mice infected with the mce1 operon mutant (Shimono et al. 2003), which is constitutively diminished in its ability to import FM (Dunphy et al. 2010), is an example of a clinical outcome that may be due to the cell wall lipid changes in this mutant. (AG: arabinogalactan layer; PG: peptidoglycan layer; CM: cytoplasmic membrane; SL-1: sulfoglycolipid-1; TMM: trehalose monomycolate; TDM: trehalose dimycolate).