Figure 2.
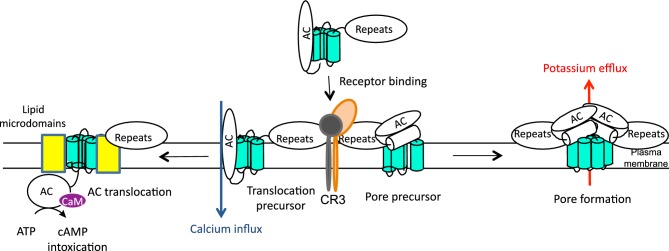
Schematic representation of CyaA action. CyaA targets primarily the host myeloid phagocytes that express the β2 integrin CD11b/CD18, known as complement receptor 3 (CR3) or Mac-1. The cell-invasive and pore-forming activities of CyaA appear to be independent and operating in parallel in target cell membrane. The current model predicts that two distinct CyaA conformers insert into target cell membrane. One would be the translocation precursor that would account for delivery of the AC domain across the lipid bilayer and provokes also a concomitant influx of calcium ions into cells. The other conformer would form a pore precursor that would oligomerize into CyaA pores, provoking potassium efflux from target cells. These two activities of a single polypeptide would then be to large extent mutually exclusive, being accomplished by the two conformer species forming in parallel and existing in an equilibrium that can be shifted in favor of prevalence of either of the conformers by alterations of CyaA acylation status, temperature, free calcium concentration, antibody binding or by specific residue substitutions (Rogel and Hanski 1992; Betsou, Sebo and Guiso 1993; Rose et al. 1995; Gray et al. 1998, 2001; Osickova et al. 1999, 2010; Rhodes et al. 2001; Basler et al. 2007).
