Abstract
Mycobacterium avium subspecies paratuberculosis (MAP), the causative agent of Johne's disease (JD) in cattle, has significant impacts on the livestock industry and has been implicated in the etiology of Crohn's disease. Macrophages play a key role in JD pathogenesis, which is driven by the manipulation of host immune mechanisms by MAP. A change in the macrophage microenvironment due to pathogenic or host-derived stimuli can lead to classical (M1) or alternative (M2) polarization of macrophages. In addition, prior exposure to antigenic stimuli has been reported to alter the response of macrophages to subsequent stimuli. However, macrophage polarization in response to MAP exposure and its possible implications have not been previously addressed. In this study, we have comprehensively examined monocyte/macrophage polarization and responsiveness to antigens from MAP-exposed and unexposed animals. At 3 years post-exposure, there was a heterogeneous macrophage activation pattern characterized by both classical and alternate phenotypes. Moreover, subsequent exposure of macrophages from MAP-exposed cattle to antigens from MAP and other mycobacterial species led to significant variation in the production of nitric oxide, interleukin-10 and tumour necrosis factor α. These results indicate the previously unreported possibility of changes in the activation state and responsiveness of circulating monocytes/macrophages from MAP-exposed cattle.
Keywords: IL-10, Johne's disease, macrophage polarization, Mycobacterium avium subspecies paratuberculosis, nitric oxide, TNF-α, tolerance
Changes in the activation of a key immune cell type, the macrophage, are evident in animals that have been exposed to the mycobacteria that causes Johne's disease.
Graphical Abstract Figure.
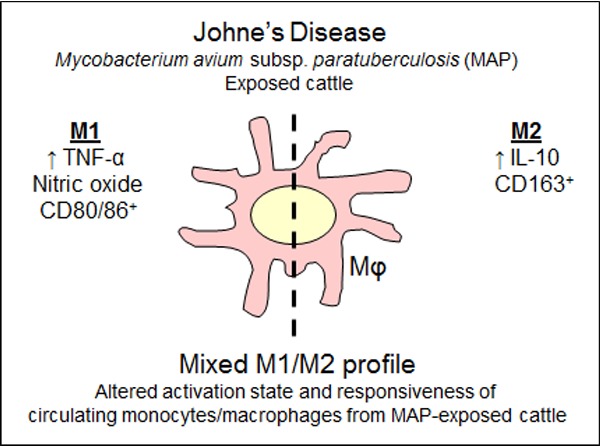
Changes in the activation of a key immune cell type, the macrophage, are evident in animals that have been exposed to the mycobacteria that causes Johne's disease.
INTRODUCTION
Johne's disease (JD) is caused by Mycobacterium avium subspecies paratuberculosis (MAP) and affects ruminants such as cattle and sheep. The scrutiny on this organism is a result of the economic loss to the livestock industry due to reduced production and public health concerns due to the notion of MAP as an aetiological agent of Crohn's disease in humans (Greenstein and Collins 2004; Hasonova and Pavlik 2006; Raizman Fetrow and Wells 2009). Infection with MAP is characterized by its chronicity, which is potentiated by survival tactics employed by the pathogen to cause alterations in the immune machinery and thereby evade the onslaught of host immune responses that are elicited (Stabel 2006). As the target cell for MAP intracellular infection, the macrophage has been extensively studied mainly using in vitro infection models, with alterations in the function of these cells being reported (Weiss et al. 2005; Janagama et al. 2006; Kabara et al. 2010).
Macrophage phenotype can be characterized based on distinct functional properties (Mills et al. 2000). In response to the cytokine profile of the microenvironment, pathogenic challenge and/or cellular interactions, macrophages can differentiate into classically activated M1 or alternatively activated M2 cells that differ in their surface receptor expression, cytokine and chemokine production (Benoit, Desnues and Mege 2008). M1 macrophages are microbicidal, proinflammatory and responsive to IFN-γ while M2 macrophages are poorly microbicidal and have anti-inflammatory properties (Mosser and Edwards 2008). A wide range of markers can be used to identify cell surface proteins representing specific macrophage phenotypes. The antigen presentation and costimulatory molecules, MHC class II, CD80 and CD86, are increased in macrophages with an M1 profile (Benoit, Desnues and Mege 2008) while surface expression of IL-1 receptor alpha and CD163, a scavenger receptor, are associated with an M2 profile (Oliveira, McClellan and Hansen 2010). In addition, M1 macrophages also express relatively large amounts of iNOS, resulting in increased production of nitric oxide, and express proinflammatory cytokines including interleukin (IL)-12, IL-23 and tumour necrosis factor-alpha (TNF-α). Conversely, M2 macrophages express higher levels of arginase-1 with resultant reduced nitric oxide production and have increased production of anti-inflammatory cytokines such as IL-10 (Mosser and Edwards 2008). Functional polarization of bovine macrophages in response to M1 and M2 stimuli has been recently reported (Castillo-Velázquez et al. 2013).
Manipulation of macrophage polarization is regarded as a factor in the survival strategies employed by intracellular pathogens such as mycobacteria (Plueddemann et al. 2011). Previous studies examining macrophage polarization in mycobacterial infections have focused on M. tuberculosis, M. leprae and M. bovis (Kahnert et al. 2006; Montoya et al. 2009; Andrade et al. 2012; Castillo-Velázquez et al. 2013). A comparison of common responses with bacterial pathogens at the transcriptomic level encompassing different bacterial species and across studies has shown that genes associated with an M1 pattern of activation are most commonly expressed (Benoit, Desnues and Mege 2008). Mycobacteria have been found to interfere with and inhibit M1 polarization by favouring M2 polarization and the resultant dampening of pro-inflammatory responses, mediated by anti-inflammatory cytokines such as IL-10, could contribute to disease persistence (Nagabhushanam et al. 2003; Miller et al. 2004; Schreiber et al. 2009). However, a recent study has shown that the macrophage activation profiles in vivo in response to virulent mycobacteria may be more complex (Andrade et al. 2012).
IFN-γ is required to survive mycobacterial infections, as identified by gene knockout studies in mice and human case studies of mycobacterial infections in patients with a defect in the IFN-γ pathway (Flynn et al. 1993; Bustamante et al. 2014). The complex role that IFN-γ plays in mycobacterial disease immunity is not yet fully understood; importantly, the temporal pattern and size of the IFN-γ response are required to generate protection (Hope et al. 2011; de Silva et al. 2013). Increased IFN-γ production has been associated with protective immunity to mycobacterial infections including paratuberculosis, and reduced IFN-γ signalling has been associated with bovine monocytes infected with MAP (Stabel 2006; Arsenault et al. 2012; de Silva et al. 2013). Longitudinal IFN-γ responses in cattle with paratuberculosis have not been reported; however, a study in sheep has shown an association between reduced antigen-specific IFN-γ responses early post-exposure and paratuberculosis disease susceptibility (de Silva et al. 2013). Understanding the complexity of the immune responses to MAP-exposure and the interactions between innate and adaptive immunity is important to understand pathogenesis and also to devise optimum control strategies such as early diagnosis and effective vaccination.
We report here the first comprehensive study to assess macrophage polarization and responsiveness associated with MAP exposure. In order to determine if the functional characteristics of macrophages are altered in vivo, the surface expression of molecular markers and production of effector agents namely nitric oxide, TNF-α and IL-10 in response to stimulation with mycobacteria and mycobacterial antigens was examined. We provide evidence for the existence of a heterogeneous monocyte/macrophage population with a mixed M1/M2 phenotype and differential responsiveness to mycobacterial antigens in macrophages from cattle exposed to MAP, which were predominantly independent of an animal's potential to secrete high levels of IFN-γ during the acute stages of MAP exposure. These findings may have implications for disease outcome and vaccination efficacy in JD.
MATERIALS AND METHODS
Animals and sampling of tissues
Ethical clearance for all animal procedures was obtained from the Animal Ethics Committee, University of Sydney. Male Holstein and Holstein/Australian Red cross calves were selected from a property in New South Wales that was unexposed to MAP and both the dams and calves were confirmed to be negative by faecal culture and faecal PCR for MAP (Plain et al. 2014). The experimental inoculation protocol was similar to a validated ovine model (Begg et al. 2010) but using a cattle (C) strain of MAP for inoculating the animals (Purdie et al. 2012). The selected calves were 3–4 months of age when administered with a low-passage number laboratory seed stock of MAP (CM00/416/C4). The inoculation dose was determined using the most probable number method, as previously described (Reddacliff et al. 2003). Calves were inoculated with three doses over a period of 1 month with the total dose of viable MAP administered estimated at 9.46 × 109. The control unexposed group consisted of 10 age-matched calves, with exposed and unexposed cohorts maintained in different paddocks at the University of Sydney Camden farms to prevent cross-contamination. Faecal and blood samples were collected monthly for the first 6 months and then every 2–3 months. Culture to detect viable MAP in the faeces was performed as previously described (Whittington et al. 1998, 1999). MAP-specific IFN-γ responses from whole blood were assessed as previously described (Begg et al. 2009). Blood samples for the isolation of monocyte-derived macrophages were collected at 3 years post-inoculation.
Preparation of bovine monocyte-derived macrophages
Isolation of PBMCs was carried out by density gradient centrifugation (de Silva et al. 2010). Briefly, blood was collected from the jugular vein into lithium heparin-coated tubes (Vaccutainer; BD Biosciences, San Jose, CA), spun at 1544 x g for 20 min and the buffy coat cells harvested. These were diluted 1:3 in PBS and layered over Ficoll Paque Plus (GE Healthcare Bio-Sciences, Uppsala, Sweden), and then centrifuged for 30 min at 754 x g without brake. Immunomagnetic separation of CD14+ monocytic cells from PBMC was carried out using anti-human CD14 antibody (TUK4) coated beads (Miltenyi Biotec, Bergish Gladbach, Germany), known to cross-react with bovine CD14, according to the manufacturer's instructions. Flow cytometric analysis to confirm the purity of the positively selected population was carried out on the pre-separation and positively selected populations. Isolated bovine monocytes were resuspended in Macrophage serum-free culture medium (Macrophage SFM) (Thermo Fisher Scientific, Rockford, IL) supplemented with recombinant human Macrophage Colony Stimulating Factor (Sigma-Aldrich) (1 ng/mL), which has reactivity across multiple mammalian species (Francey et al. 1992; Gow et al. 2012).
Flow cytometry
Freshly isolated monocytes were resuspended in FACS buffer (PBS with 2% newborn calf serum and 0.05% sodium azide) and plated in 96-well plates at 1 × 105 cells/well. The cells were then stained with primary antibodies or matching isotype controls (Table 1). Cells were incubated for 10 min in the dark followed by washing twice with FACS buffer by centrifugation at 233 x g for 10 min, and then staining with a secondary antibody if required. Labelled cell populations were fixed with 1% paraformaldehyde. Flow cytometric data was acquired on a FACSCalibur flow cytometer and analysed using CellQuest Pro software (BD Biosciences). A marker was applied that defined the positively stained population and expression of CD80, CD86 and CD163 assessed. Data are presented as the percentage of positively stained cells for each individual antibody, after subtraction of the corresponding value for the isotype control antibody-stained population.
Table 1.
Primary, secondary and isotype control antibodies for flow cytometry.
| Target molecule | Conjugate | Clone | Isotype |
|---|---|---|---|
| Primary antibody | |||
| CD14a | FITC | TUK4 | IgG2a |
| MHC class IIb | None | H42A | IgG2a |
| CD11ba | None | CC126 | IgG2b |
| CD163a | None | 2A 10/11 | IgG1 |
| CD5d | FITC | CC17 | IgG1 |
| B-B4 (B-cell marker)b | None | BAQ155A | IgG1 |
| γδ T-cell receptord | FITC | 86D | IgG1 |
| CD80c | FITC | BB1 | IgM,κ |
| CD86c | None | IT2.2 | IgG2b,κ |
| Secondary/ isotype control antibody | |||
| Anti-mouse IgG1c | APC | X56 | IgG1,κ |
| Anti-mouse IgG2be | APC | Polyclonal | – |
| Anti-mouse IgG2af | APC | Polyclonal | – |
| Mouse isotype IgG2ac | FITC | G155-178 | Mouse IgG2a,κ |
| Rat anti-mouse IgMc | FITC | II41 | Rat IgG2a,κ |
aSupplied by ABD Serotec,Oxford,UK; bsupplied by VMRD Inc, Pullman, WA; csupplied by BD Pharmingen, San Diego, CA; dprimary antibodies were produced from the cell lines (86D was the kind gift of Dr Garry Barcham, University of Melbourne) and conjugated to FITC using a Fluroreporter FITC labelling kit (Molecular probes); esupplied by Columbia Biosciences, Columbia, MD; fsupplied by Caltag Lab, Burlingame, CA.
Macrophage stimulation cultures
Monocytes were plated at 1×105 cells/well (100 μL) into 96-well flat-bottom plates (BD) and cultured for 8–10 days at 37°C in a humidified atmosphere of 5% CO2 in air until confluent macrophage monolayers were achieved. For stimulation cultures, LPS from E. coli (Sigma-Aldrich) was used as the positive control antigen at a final concentration of 25 ng/mL. Both the French pressed MAP 316v strain whole-cell-derived antigen (MAP 316v antigen) and M. bovis purified protein derivative (PPDB) were used at a final concentration of 100 μg/mL. The C strain of MAP CM00/416 (Marsh et al. 2006) and M. smegmatis (a kind gift from Dr Nicolas West, University of Sydney) were used live or heat-killed (80°C for 1 h) at an MOI of 1:1.
Some macrophage cultures were pre-treated with recombinant bovine IFN-γ (Thermo Fisher Scientific) at a concentration of 100 ng/mL for a period of 1 h prior to antigen stimulation. Cells were then washed with fresh culture medium and both the untreated and IFN-γ pre-treated cells were incubated with antigens/bacteria at 37°C in a humidified atmosphere of 5% CO2 in air and supernatants were harvested at 48 h post-incubation. All samples were tested in triplicate for the nitric oxide assay or in duplicate for the cytokine assays.
Cytokine and nitric oxide assays
MAP-specific IFN-γ production was assessed on plasma following a whole blood stimulation culture (48 h) with MAP 316v antigen, as previously described (Begg et al. 2009). The MAP-exposed cattle were categorized based on their IFN-γ response at 4 months post-inoculation as high (an IFN-γ ELISA S/P ratio of 0.4–0.9) (n = 5) or low (an IFN-γ ELISA S/P ratio of 0.2-0.3) (n = 5) responders and followed throughout the trial (Fig. 1).
Figure 1.
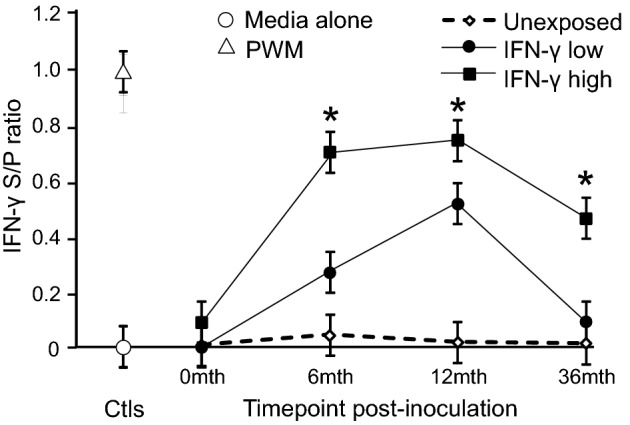
MAP-specific whole-blood IFN-γ responses from exposed and unexposed cattle at 0, 6, 12 and 36 months post-inoculation. Results are the IFN-γ sample to positive (S/P) ratio mean ± standard error of the mean derived from REML linear mixed model. The MAP-exposed cattle were categorized based on their IFN-γ response at 4 months post-inoculation as high (an IFN-γ ELISA S/P ratio of 0.4–0.9) (n = 5) or low (an IFN-γ ELISA S/P ratio of 0.2–0.3) (n = 5) responders and grouped accordingly. Asterisk indicates significantly higher IFN- γ responses (P < 0.05) compared to low responders and unexposed control groups. Negative stimulation control (Media alone) and positive control pokeweed mitogen (PWM) responses are averaged across all animals and timepoints.
Nitric oxide production in macrophage cultures was estimated by the Griess assay (Sigma-Aldrich), according to the manufacturer's instructions. The IL-10 response was assessed in supernatants of cultured macrophages by ELISA (Kwong et al. 2002; de Silva, Begg and Whittington 2011) and the TNF-α response was estimated using the bovine TNF-α VetSet ELISA development kit (Kingfisher Biotech, Saint Paul, MN) according to the manufacturer's protocol.
Statistical analysis
Data were analysed for differences between pre-treatment groups, subgroups and between the various stimulants used by restricted maximum likelihood (REML) analysis in a linear mixed model (GenStat, 13th edition, VSN International Ltd, UK), with statistical significance set at the 5% level. The linear mixed model as fitted using the REML procedure caters for correlated data as a result of observations being taken from the same animal at multiple time points as has been done for the IFNγ responses in blood. REML also accounts for the degrees of freedom lost by estimating the fixed effects, and makes an unbiased estimation of random effects variances and hence is considered to be a stringent model for estimating variance components in an experiment. Predicted means were generated by the model and were considered significantly different to each other if they varied by an amount greater than the least square of differences.
RESULTS
Bovine infection model and MAP-specific IFN-gamma responses
Calves experimentally inoculated with viable MAP organisms were compared with age-matched control unexposed cattle. The MAP-specific blood IFN-γ responses of the exposed calves were examined monthly for the first 6 months and then every 2–3 months and the animals subgrouped into early high and low IFN-γ responders; the IFN-γ response was significantly different between these two groups throughout the trial (Fig. 1). The circulating monocytes from these exposed and unexposed cattle were examined at 3 years post-inoculation. Following inoculation, intermittent shedding of MAP was detected in the faeces of the exposed cattle by culture (data not shown); however, none were consistently shedding MAP in their faeces at the time of sampling. This is consistent with the time course of the disease in cattle, with clinical signs generally not evident for many years post-exposure.
Monocytes from MAP-exposed cattle express CD80, CD86 and CD163
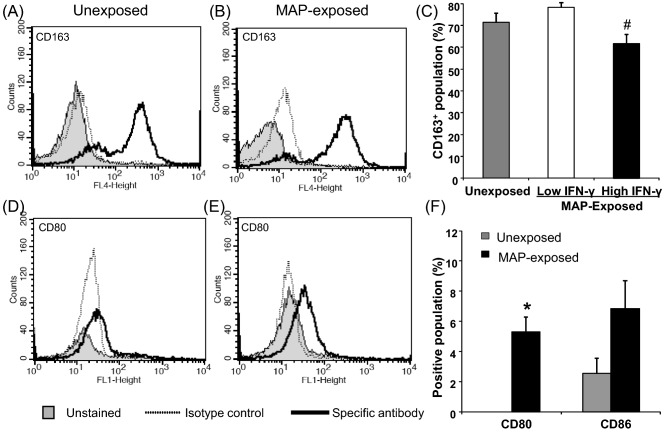
Monocytes were isolated from peripheral blood using anti-CD14 antibody-coated microbeads and confirmed by flow cytometry to be 92–95% CD14+ and have phenotypic markers associated with monocytic cells; MHC class II+, CD11b+ and negative for CD5, B-B4 (B-cell marker) and γδ T-cell receptor (data not shown). The majority of the isolated monocyte cell population in both unexposed and MAP-exposed cattle was CD163 positive (Fig. 2 A–C). There was no significant difference between the CD163 expression level in unexposed and MAP-exposed cattle. However, when the MAP-exposed cattle were subgrouped into those with early high and low IFN-γ responses, the proportion of cells that expressed CD163 was significantly decreased (P ≤ 0.05) in the high IFN-γ responders in comparison with the low responders (Fig. 2C). A higher proportion of monocytes isolated from MAP-exposed cattle expressed CD80 and CD86 in comparison with monocytes from unexposed control cattle, although this was significant only for CD80 (P ≤ 0.05) (Fig. 2F). This was observed as a shift in the population to the right (flow cytometric peak shift) (Fig. 2D and E). There were no significant differences in expression of CD80 or CD86 between the cattle with high and low IFN-γ responses (data not shown). The expression of M1 and M2 cell surface markers on unexposed and MAP-exposed monocytes is summarized in Table 2.
Figure 2.
Expression of CD163, CD80 and CD86 by monocytes from unexposed and MAP-exposed cattle. PBMC were isolated from the whole blood of unexposed control (n = 5), MAP-exposed (n = 10) cattle by Ficoll-density gradient separation. The MAP-exposed cattle were selected based on either low blood IFN-γ responses (n = 5) or high IFN-γ responses (n = 5). CD14+ selection by the MACS® isolation system was used to separate the monocytic population. Staining for M2 macrophage marker CD163 is shown in panels A, B and C and costimulatory molecules, CD80 and CD86, are shown in panels D, E and F. Representative flow cytometry profiles are shown for CD163 expression by monocytes from unexposed (A) and MAP-exposed (B) cattle and CD80 expression by monocytes from unexposed (D) and MAP-exposed (E) cattle. The shaded histogram is the unstained population, the dotted line is the staining for the isotype control and the thick black line is the population staining for the specific antibody. CD86 profiles are not shown but there was a similar population profile as seen for CD80. Panels C and F show combined surface molecular staining data for monocytes from all unexposed and MAP-exposed cattle. Data are the mean positive population (%) plus standard error of the mean for replicate stained wells of monocytes from 5 control unexposed and 10 MAP-exposed cattle; for CD163 these were divided into low and high IFN-γ responder subgroups. Gating of the positive population was based on the isotype control antibody staining. Hash denotes a significant decrease (P ≤ 0.05) in CD163 expression MAP-exposed cattle with high and low IFN- γ responses. Asterisk denotes a significant increase (P ≤ 0.05) in the CD80 expression in monocytes from the MAP-exposed cattle in comparison with the unexposed control animals.
Table 2.
Expression of monocyte/macrophage surface markers and effector molecules associated with an M1 or M2 phenotype in unexposed and MAP-exposed cattle.
| MAP-exposed | |||||
|---|---|---|---|---|---|
| Surface marker/ | M1 or | IFN-γ | IFN-γ | ||
| effector moleculea | M2b | Unexposed | All | low | high |
| CD80 | M1 | – | + | + | + |
| CD86 | M1 | + | ++ | +++ | ++ |
| Nitric oxide | M1 | ++ | ++ | + | ++ |
| TNF-α | M1 | + | ++++ | ++++ | ++++ |
| CD163 | M2 | +++ | +++ | +++ | +++ |
| IL-10 | M2 | + | ++ | ++ | ++ |
aFor effector molecules, the responses to MAP and/or MAP Ag are represented.
bDenotes whether a marker/molecule is generally associated with an M1 or an M2 phenotype, based on the available literature in other species.
Nitric oxide production by monocyte-derived macrophages from exposed cattle
To assess the ability of monocyte-derived macrophages from MAP-exposed cattle to respond to stimulants, including MAP and its antigens, monocytes were isolated from the peripheral blood of MAP-exposed and unexposed cattle, cultured for 8–10 days and their nitric oxide production assessed following stimulation (Fig. 3). Cultures were stimulated with MAP 316v antigen, M. bovis antigen (PPDB) or LPS as a positive control. Additionally, the ability to respond to live MAP, and the non-pathogenic mycobacterium, M. smegmatis, was compared. The role of IFN-γ was examined by comparing cellular responses with and without pre-stimulation with bovine IFN-γ.
Figure 3.
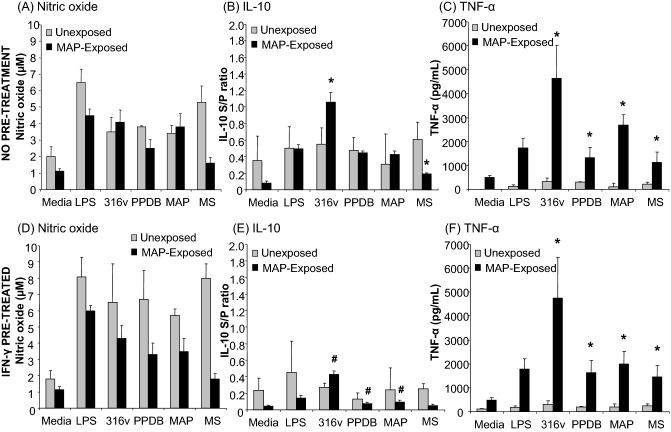
Nitric oxide and cytokine responses of monocyte-derived macrophages to mycobacteria and antigens. PBMCs were isolated from the whole blood of unexposed control (n = 5), MAP-exposed (n = 10) cattle and monocytes isolated by CD14+ selection using MACS®. Cultured bovine macrophages were incubated for a period of 48 h with LPS (25 ng/mL), MAP 316v antigen (316v) or M. bovis antigen (PPDB; 100 μg/mL), live MAP or M. smegmatis strains at an MOI of 1:1 (viable count). Cells were cultured without pre-treatment (top panels A–C) or after IFN-γ pre-treatment for 1 h (lower panels D–F). Supernatants were assayed for production of nitric oxide (A, D); IL-10 (B, E) or TNF-α (C, F). Data are the mean plus standard error of the mean of three replicate cultures from one out of two independent experiments. Asterisk denotes a significant difference (P ≤ 0.05) in macrophage responses between the unexposed and MAP-exposed cattle. Hash denotes a significant difference (P ≤ 0.05) between the macrophage responses from MAP-exposed cattle without IFN-γ pre-treatment and after IFN-γ pre-treatment.
Monocyte-derived macrophages from both unexposed and MAP-exposed cattle produced nitric oxide in response to live MAP and MAP 316v antigen (Fig. 3A and D). There was no significant difference in overall nitric oxide secretion by cells from unexposed and MAP-exposed cattle, without IFN-γ pre-treatment. There was however a significant overall decrease in nitric oxide production across all treatment groups by macrophages from the MAP-exposed cattle in comparison to the unexposed control cattle in those cultures pre-treated with IFN-γ (P ≤ 0.05). Differences between individual treatment groups for this effector agent did not reach significance.
When the exposed cattle were subdivided into low and high IFN-γ responders, it was observed that monocyte-derived macrophages from exposed animals with a high MAP-specific IFN-γ response had a significantly higher (P ≤ 0.05) nitric oxide response to live MAP, in comparison with the low IFN-γ responders (Fig. 4). This was the case for cultures with or without IFN-γ pre-treatment.
Figure 4.
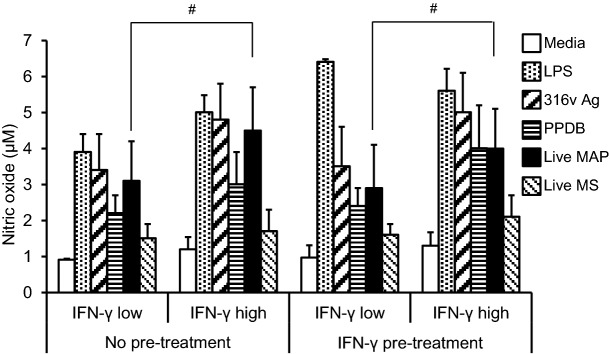
Nitric oxide responses of monocyte-derived macrophages cultured from MAP-exposed cattle, subgrouped into low and high IFN-γ responders. PBMC were isolated from the whole blood from MAP-exposed (n = 10) cattle and monocytes isolated by CD14+ selection using MACS®. The MAP-exposed cattle were subgrouped into low (n = 5) or high IFN-γ responders (n = 5), based on responses in the whole-blood MAP-specific IFN-γ assay. Cultured bovine macrophages were incubated with LPS (25 ng/mL), MAP 316v antigen (316v Ag) or M. bovis antigen (PPDB) (100 μg/mL), live MAP or M. smegmatis strains at an MOI of 1:1 (viable count) for a period of 48 h, without pre-treatment or after IFN-γ pre-treatment for 1 h. Data are the mean plus standard error of the mean of three replicate cultures from one out of two independent experiments. Hash denotes a significant increase (P ≤ 0.05) in nitric oxide response to incubation with live MAP in the high compared to the low IFN-γ responder subgroup, with or without IFN-γ pre-treatment.
Monocyte-derived macrophages from exposed cattle produce both IL-10 and TNFα in response to MAP antigens
As with the nitric oxide studies, monocyte-derived macrophages from the peripheral blood of MAP-exposed and unexposed cattle were cultured for 8–10 days and then stimulated (Fig. 3). There was a significant increase in IL-10 production (P ≤ 0.05) in response to MAP 316v antigen by macrophages from the MAP-exposed cattle in comparison to the unexposed cattle for cultures without IFN-γ pre-treatment (Fig. 3B). Contrary to this, there was a significant decrease (P ≤ 0.05) in IL-10 production obtained in macrophages from the exposed cattle in response to live M. smegmatis in comparison with the unexposed cattle (Fig. 3B). When the cells were pre-treated with IFN-γ, the IL-10 responses overall tended to be lower and macrophages from the exposed cattle responded with a significant decrease (P ≤ 0.05) in IL-10 production in comparison to the group without any pre-treatment in response to incubation with MAP 316v antigen, PPDB and live MAP (Fig. 3E). The highest IL-10 response was seen in cultures of macrophages from MAP-exposed cattle that were stimulated with MAP 316v antigen, without IFN-γ pre-treatment (Fig. 3B and E). When the exposed cattle were subdivided into IFN-γ low and high responders, there were no significant differences in monocyte-derived macrophage IL-10 responses between the subgroups, though the significant decrease in IL-10 production in IFN-γ pre-treated cultures was seen for both subgroups (Fig. S1, Supporting Information).
There was a significant increase (P ≤ 0.05) in TNF-α production in response to both antigens and bacteria by macrophages from the MAP-exposed cattle compared to the unexposed cattle with or without IFN-γ pre-treatment (Fig. 3C and F). The positive control (LPS) did not induce an appreciable TNF-α response in macrophages from the unexposed cattle, but it was induced in macrophages from the exposed cattle. Unlike the IL-10 response, IFN-γ pre-treatment did not significantly reduce TNF-α production in the different treatment groups (Fig. 3F). The highest TNF-α level was seen in cultures of macrophages from MAP-exposed cattle that were stimulated with MAP 316v antigen, either with or without IFN-γ pre-treatment (Fig. 3C). No significant differences were seen between the subgroups of low and high IFN-γ responders with regard to TNF-α responses (Fig. S2, Supporting Information). The production of effector molecules by bovine macrophages from unexposed and MAP-exposed cattle is summarized in Table 2.
DISCUSSION
To our knowledge this is the first study reporting specifically on the issue of macrophage polarization ex vivo in JD, relative to the magnitude of the animal's response in an IFN-γ release assay. Based on the study of surface marker expression by freshly isolated monocytes as well as production of effector molecules by isolated macrophages from unexposed and MAP-exposed cattle in vitro, we show that subclinical MAP infection is characterized by the presence of both M1 and M2 activation subtypes of macrophages. The in vitro studies on cultured monocyte-derived macrophages from unexposed and MAP-exposed cattle reinforce the findings of the ex vivo study. These responses though modulated in MAP-exposed animals occur largely independent of the animal's degree of IFN-γ responsiveness to MAP antigens, detected during the early stages of infection. We also raise the question of the phenomenon of cross tolerance in MAP-infection, indicating the complexity of immune responses elicited during this disease.
Monocytes isolated from MAP-exposed cattle expressed CD80 and CD86 suggesting the presence of M1 macrophage phenotypes, with CD80 expression significantly increased compared to unexposed controls. There were no significant differences in CD163 expression, an M2 marker, by monocytes from the unexposed and MAP-exposed cattle. However, when the MAP-exposed animals were subgrouped based on their early IFN-γ responses, a significant decrease in the expression of CD163 in animals with a high IFN-γ response was observed. Collectively, these results suggest that MAP exposure does not lead to a specific polarized pattern of the circulating monocytes.
We further assessed macrophage effector functions associated with M1 and M2 profiles, namely production of nitric oxide and the cytokines, IL-10 and TNF-α. Macrophages cultured from unexposed cattle pre-treated with IFN-γ had significantly increased nitric oxide production upon stimulation with mycobacteria and antigens in comparison to those from MAP-exposed cattle. This is similar to the findings previously reported by Simutis, Jones and Hostetter (2007) who found that an IFN-γ concentration capable of activating macrophages under normal conditions was insufficient at inducing significant nitric oxide production by macrophages conditioned to MAP antigens (Simutis, Jones and Hostetter 2007), thus favouring the development of the M2 phenotype. However, increased nitric oxide production was reported after addition of live MAP to PBMC cultures from healthy cattle and cattle clinically infected with MAP, boosted by treatment with IFN-γ (Khalifeh, Al-Majali and Stabel 2009); this was not observed in the current study and may reflect differences in the cell types assessed and/or time point of sampling in relation to disease development.
The enhanced ability of macrophages from MAP-exposed cattle to produce TNF-α and IL-10 in response to MAP and/or MAP antigen supports the absence of a clearly delineated pro- or anti-inflammatory shift in the effector functions of the macrophages following MAP exposure. Unlike nitric oxide production, TNF-α and IL-10 responses did not vary between the IFN-γ high and low responders, which suggests that the differences found are more dependent on MAP exposure status per se than on the early IFN-γ responses of an animal to that exposure. The MAP 316v whole cell antigen appeared to promote stronger macrophage responses than live MAP, which may be due to differences in the availability or recognition of the MAP antigens over the time of the stimulation cultures or an inherent difference related to macrophage infection by live MAP.
Pre-treatment of macrophage cultures with IFN-γ decreased overall IL-10 production in response to antigens as well as live MAP, consistent with IFN-γ favouring a shift to a classically activated M1 phenotype. IL-10 is known to skew the macrophage phenotype toward M2, as assessed by the expression of several markers including CD163, which is considered to be highly M2 specific (Weiss et al. 2005). Linking these findings with the decreased CD163 expression ex vivo in animals with high IFN-γ responses suggests that a shift toward increased IFN-γ along with IL-10 could play a role in CD163 expression following MAP-exposure.
Tolerance in macrophages and other antigen presenting cells due to LPS or other bacterial antigens results in a state of transient hyporesponsiveness to subsequent stimulation with the same or another antigen (cross-tolerance) (Dalpke et al. 2005; Yang et al. 2012). Non-pathogenic M. smegmatis is capable of inducing the differentiation of human monocytes into mature dendritic cells and it was concluded that this might be a plausible mechanism by which environmental mycobacteria influence immune responses to pathogenic species (Martino et al. 2005). The findings of Martino et al. (2005) are reflected in our study, wherein M. smegmatis caused enhanced nitric oxide and IL-10 in unexposed cattle only and prior exposure to MAP appears to have influenced subsequent macrophage responses to this mycobacterium. This suggests that there is the potential for cross-tolerance between non-pathogenic environmental strains like M. smegmatis and pathogenic mycobacteria such as MAP, which may influence immunity and subsequent macrophage responses. This finding needs to be verified by further studies. Regardless, the phenomenon has important implications in addressing issues associated with vaccine efficacy, as it has been shown that tolerance due to exposure to environmental mycobacteria could interfere with the efficacy to BCG vaccine (Flaherty et al. 2006; Young et al. 2007).
Our study does have limitations with regard to the monocyte isolation method, culture conditions as well as reagent choice. Monocytes used in this study were positively selected using microbeads targeting CD14, which has long been considered as a specific marker for monocyte/macrophages including those of bovine origin (Seo et al. 2009; Machugh et al. 2012). A recently published study reported a minor population of circulating bovine monocytes that were CD16+ and expressed low levels of CD14 (Hussen et al. 2013), similar to the monocyte subsets found in humans (Ziegler-Heitbrock 2007). This information was not available while our study was being conducted and it is possible that selection based on only the CD14+ marker could have led to an intrinsic bias in the findings of this study. We cultured the macrophages in tissue culture plates to fully reflect the mature nature of the macrophage in a tissue microenvironment where adherence is essential. While these factors might lead to a bias in the expression level of certain markers, our results are based on a comparison of the MAP-exposed animals with the unexposed controls.
However, given these limitations, the power of this study is the fact that outbred animals were examined, using an infection model in the natural host, to demonstrate significant phenotypic and functional differences of monocytes/macrophages from MAP-exposed animals. It would be of interest to examine macrophage populations derived directly from lesions in the gut and associated lymph nodes to determine if the heterogeneous macrophage activation is present locally, as cells from the circulation may not reflect the situation at the intestinal site due to compartmentalization in the generated immune responses (Koets, Eda and Sreevatsan 2015).
In conclusion, circulating monocytes from MAP-exposed cattle express CD163 as well as CD80 and CD86 and in vitro these monocyte-derived macrophages have responses characteristic of both classical proinflammatory M1 cells and alternatively activated, anti-inflammatory M2 cells. These changes are predominantly independent of whether the animals were high or low responders for the protective cytokine, IFN-γ. We raise the question of the possible existence of the phenomenon of macrophage cross-tolerance in mycobacterial infection, which may be of importance when viewed from the perspective of vaccination. Currently, there are no studies addressing whether MAP vaccination or prior exposure to related organisms could cause immune tolerance that may impact on the ability of vaccination to protect animals against disease.
Supplementary Material
Acknowledgments
The authors would like to thank Mrs. Anna Waldron, Ms. Nicole Carter, Mr. Craig Kristo, Mr. Nobel Toribio, Mr. Lee White and Mr. James Dalton for sample preparation, laboratory and animal husbandry support and Dr Navneet Dhand for advice on statistical analysis.
SUPPLEMENTARY DATA
FUNDING
This work was supported by Meat and Livestock Australia and Cattle Council of Australia, Sheep Meat Council of Australia, Wool Producers Australia through Animal Health Australia.
Conflict of interest. None declared.
REFERENCES
- Andrade M, Amaral EP, Ribeiro S, et al. Pathogenic Mycobacterium bovis strains differ in their ability to modulate the proinflammatory activation phenotype of macrophages. BMC Microbiol. 2012;12:166. doi: 10.1186/1471-2180-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault RJ, Li Y, Bell K, et al. Mycobacterium avium subsp. paratuberculosis inhibits gamma interferon-induced signaling in bovine monocytes: insights into the cellular mechanisms of Johne's disease. Infect Immun. 2012;80:3039–48. doi: 10.1128/IAI.00406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg DJ, de Silva K, Bosward K, et al. Enzyme-linked immunospot: an alternative method for the detection of interferon gamma in Johne's disease. J Vet Diagn Invest. 2009;21:187–96. doi: 10.1177/104063870902100202. [DOI] [PubMed] [Google Scholar]
- Begg DJ, de Silva K, Di Fiore L, et al. Experimental infection model for Johne's disease using a lyophilised, pure culture, seedstock of Mycobacterium avium subspecies paratuberculosis. Vet Microbiol. 2010;141:301–11. doi: 10.1016/j.vetmic.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Bustamante J, Boisson-Dupuis S, Abel L, et al. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol. 2014;26:454–70. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Velázquez U, Gomez-Flores R, Tamez-Guerra R, et al. Differential responses of macrophages from bovines naturally resistant or susceptible to Mycobacterium bovis after classical and alternative activation. Vet Immunol Immunop. 2013;154:8–16. doi: 10.1016/j.vetimm.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Dalpke AH, Lehner MD, Hartung T, et al. Differential effects of CpG-DNA in Toll-like receptor-2/-4/-9 tolerance and cross-tolerance. Immunology. 2005;116:203–12. doi: 10.1111/j.1365-2567.2005.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva K, Begg D, Whittington R. The interleukin 10 response in ovine Johne's disease. Vet Immunol Immunop. 2011;139:10–6. doi: 10.1016/j.vetimm.2010.07.022. [DOI] [PubMed] [Google Scholar]
- de Silva K, Begg D, Carter N, et al. The early lymphocyte proliferation response in sheep exposed to Mycobacterium avium subsp. paratuberculosis compared to infection status. Immunobiology. 2010;215:12–25. doi: 10.1016/j.imbio.2009.01.014. [DOI] [PubMed] [Google Scholar]
- de Silva K, Begg DJ, Plain KM, et al. Can early host responses to mycobacterial infection predict eventual disease outcomes? Prev Vet Med. 2013;112:203–12. doi: 10.1016/j.prevetmed.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Flaherty DK, Vesosky B, Beamer GL, et al. Exposure to Mycobacterium avium can modulate established immunity against Mycobacterium tuberculosis infection generated by Mycobacterium bovis BCG vaccination. J Leukocyte Biol. 2006;80:1262–71. doi: 10.1189/jlb.0606407. [DOI] [PubMed] [Google Scholar]
- Flynn JL, Chan J, Triebold KJ, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francey T, Jungi TW, Rey O, et al. Culture of ovine bone marrow-derived macrophages and evidence for serum factors distinct from M-CSF contributing to their propagation in vitro. J Leukocyte Biol. 1992;51:525–34. doi: 10.1002/jlb.51.6.525. [DOI] [PubMed] [Google Scholar]
- Gow DJ, Garceau V, Kapetanovic R, et al. Cloning and expression of porcine Colony Stimulating Factor-1 (CSF-1) and Colony Stimulating Factor-1 Receptor (CSF-1R) and analysis of the species specificity of stimulation by CSF-1 and Interleukin 34. Cytokine. 2012;60:793–805. doi: 10.1016/j.cyto.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein RJ, Collins MT. Emerging pathogens: is Mycobacterium avium subspecies paratuberculosis zoonotic? Lancet. 2004;364:396–7. doi: 10.1016/S0140-6736(04)16781-0. [DOI] [PubMed] [Google Scholar]
- Hasonova L, Pavlik I. Economic impact of paratuberculosis in dairy cattle herds: a review. Vet Med. 2006;51:193–211. [Google Scholar]
- Hope JC, Thom ML, McAulay M, et al. Identification of surrogates and correlates of protection in protective immunity against Mycobacterium bovis infection induced in neonatal calves by vaccination with M. bovis BCG Pasteur and M. bovis BCG Danish. Clin Vaccine Immunol. 2011;18:373–9. doi: 10.1128/CVI.00543-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussen J, Duvel A, Sandra O, et al. Phenotypic and functional heterogeneity of bovine blood monocytes. PLoS One. 2013;8:e71502. doi: 10.1371/journal.pone.0071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janagama H, il Jeong K, Kapur V, et al. Cytokine responses of bovine macrophages to diverse clinical Mycobacterium avium subspecies paratuberculosis strains. BMC Microbiol. 2006;6:10. doi: 10.1186/1471-2180-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabara E, Kloss CC, Wilson M, et al. A large-scale study of differential gene expression in monocyte-derived macrophages infected with several strains of Mycobacterium avium subspecies paratuberculosis. Brief Funct Genomics. 2010;9:220–37. doi: 10.1093/bfgp/elq009. [DOI] [PubMed] [Google Scholar]
- Kahnert A, Seiler P, Stein M, et al. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36:631–47. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- Khalifeh MS, Al-Majali AM, Stabel JR. Role of nitric oxide production in dairy cows naturally infected with Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunop. 2009;131:97–104. doi: 10.1016/j.vetimm.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Koets AP, Eda S, Sreevatsan S. The within host dynamics of Mycobacterium avium ssp. paratuberculosis infection in cattle: where time and place matter. Vet Res. 2015;46:61. doi: 10.1186/s13567-015-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong LS, Hope JC, Thom ML, et al. Development of an ELISA for bovine IL-10. Vet Immunol Immunop. 2002;85:213–23. doi: 10.1016/s0165-2427(02)00007-7. [DOI] [PubMed] [Google Scholar]
- Machugh DE, Taraktsoglou M, Killick KE, et al. Pan-genomic analysis of bovine monocyte-derived macrophage gene expression in response to in vitro infection with Mycobacterium avium subspecies paratuberculosis. Vet Res. 2012;43:25. doi: 10.1186/1297-9716-43-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh IB, Bannantine JP, Paustian ML, et al. Genomic comparison of Mycobacterium avium subsp. paratuberculosis sheep and cattle strains by microarray hybridization. J Bacteriol. 2006;188:2290–3. doi: 10.1128/JB.188.6.2290-2293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino A, Sacchi A, Volpe E, et al. Non-pathogenic Mycobacterium smegmatis induces the differentiation of human monocytes directly into fully mature dendritic cells. J Clin Immunol. 2005;25:365–75. doi: 10.1007/s10875-005-4188-x. [DOI] [PubMed] [Google Scholar]
- Miller BH, Fratti RA, Poschet JF, et al. Mycobacteria inhibit nitric oxide synthase recruitment to phagosomes during macrophage infection. Infect Immun. 2004;72:2872–8. doi: 10.1128/IAI.72.5.2872-2878.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. [Google Scholar]
- Montoya D, Cruz , Teles RMB D, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe. 2009;6:343–53. doi: 10.1016/j.chom.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushanam V, Solache A, Ting L-M, et al. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-Î3. J Immunol. 2003;171:4750–7. doi: 10.4049/jimmunol.171.9.4750. [DOI] [PubMed] [Google Scholar]
- Oliveira LJ, McClellan S, Hansen PJ. Differentiation of the endometrial macrophage during pregnancy in the cow. PLoS One. 2010;5:e13213. doi: 10.1371/journal.pone.0013213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plain KM, Marsh IB, Waldron AM, et al. High-throughput direct fecal PCR assay for detection of Mycobacterium avium subsp. paratuberculosis in sheep and cattle. J Clin Microbiol. 2014;52:745–57. doi: 10.1128/JCM.03233-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plueddemann A, Mukhopadhyay S, Gordon S. Innate immunity to intracellular pathogens: macrophage receptors and responses to microbial entry. Immunol Rev. 2011;240:11–24. doi: 10.1111/j.1600-065X.2010.00989.x. [DOI] [PubMed] [Google Scholar]
- Purdie AC, Plain KM, Begg DJ, et al. Expression of genes associated with the antigen presentation and processing pathway are consistently regulated in early Mycobacterium avium subsp. paratuberculosis infection. Comp Immunol Microb. 2012;35:151–62. doi: 10.1016/j.cimid.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Raizman EA, Fetrow JP, Wells SJ. Loss of income from cows shedding Mycobacterium avium subspecies paratuberculosis prior to calving compared with cows not shedding the organism on two Minnesota dairy farms. J Dairy Sci. 2009;92:4929–36. doi: 10.3168/jds.2009-2133. [DOI] [PubMed] [Google Scholar]
- Reddacliff LA, Nicholls PJ, Vadali A, et al. Use of growth indices from radiometric culture for quantification of sheep strains of Mycobacterium avium subsp. paratuberculosis. Appl Environ Microb. 2003;69:3510–6. doi: 10.1128/AEM.69.6.3510-3516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber T, Ehlers S, Heitmann L, et al. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183:1301–12. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo KS, Park JY, Davis WC, et al. Superantigen-mediated differentiation of bovine monocytes into dendritic cells. J Leukocyte Biol. 2009;85:606–16. doi: 10.1189/jlb.0608338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simutis FJ, Jones DE, Hostetter JM. Failure of antigen-stimulated γδT cells and CD4+ T cells from sensitized cattle to upregulate nitric oxide and mycobactericidal activity of autologous Mycobacterium avium subsp. paratuberculosis-infected macrophages. Vet Immunol Immunop. 2007;116:1–12. doi: 10.1016/j.vetimm.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel JR. Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal. Anim Health Res Rev. 2006;7:61–70. doi: 10.1017/S1466252307001168. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Evanson OA, de Souza C, et al. A critical role of interleukin-10 in the response of bovine macrophages to infection by Mycobacterium avium subsp paratuberculosis. Am J Vet Res. 2005;66:721–6. doi: 10.2460/ajvr.2005.66.721. [DOI] [PubMed] [Google Scholar]
- Whittington RJ, Marsh I, McAllister S, et al. Evaluation of modified BACTEC 12B radiometric medium and solid media for culture of Mycobacterium avium subsp. paratuberculosis from sheep. J Clin Microbiol. 1999;37:1077–83. doi: 10.1128/jcm.37.4.1077-1083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington RJ, Marsh I, Turner MJ, et al. Rapid detection of Mycobacterium paratuberculosis in clinical samples from ruminants and in spiked environmental samples by modified BACTEC 12B radiometric culture and direct confirmation by IS900 PCR. J Clin Microbiol. 1998;36:701–7. doi: 10.1128/jcm.36.3.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Liu Y, Chen Y, et al. Pretreatment with Mycobacterium avium-derived lipids attenuates the response of murine macrophages to components of Mycobacterium tuberculosis. Int J Mol Med. 2012;29:1072–82. doi: 10.3892/ijmm.2012.932. [DOI] [PubMed] [Google Scholar]
- Young SL, Slobbe L, Wilson R, et al. Environmental strains of Mycobacterium avium interfere with immune responses associated with Mycobacterium bovis BCG vaccination. Infect Immun. 2007;75:2833–40. doi: 10.1128/IAI.01826-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukocyte Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.