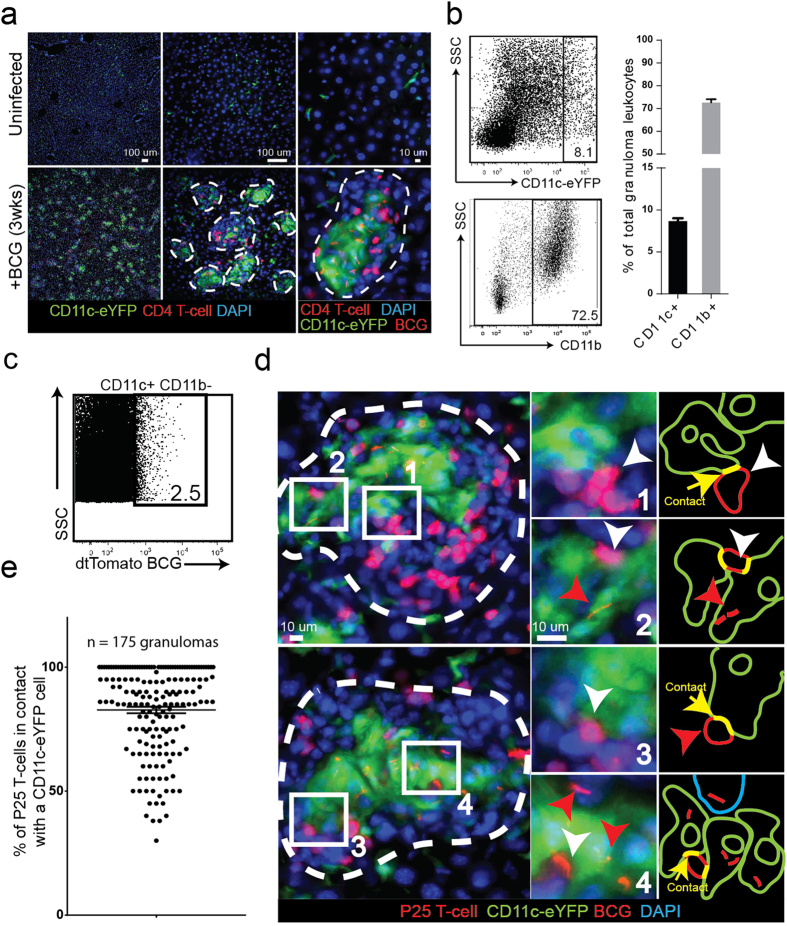
Figure 1. BCG-containing CD11c+ inflammatory dendritic cells in mycobacterial granulomas.
CD11c-eYFP reporter mice (YFP expression driven by the CD11c promoter) were IP infected with tdTomato-expressing BCG and harvested 3 weeks later during acute infection. BCG-specific DsRed P25 T-cells were transferred into infected mice one week before harvest. (A) 50 μm-thick liver sections from uninfected and infected mice showing the accumulation of BCG-containing granulomas populated by CD11c+ and P25 T-cells. Individual granulomas outlined in white dotted line. (B) Flow cytometry plots of liver-isolated granuloma cells showing the proportion of CD11c+ and CD11b+ cells in the granuloma. Bar graphs shows average value among n = 13 mice among 3 replicate experiments. (C) Granuloma cells were isolated from Wt mice infected with tdTomato BCG and stained with CD11c+ antibodies. Flow plot shows the proportion of BCG-infected CD11c+ cells (colocalization of CD11c and tdTomato signal). Plot is representative of the average value (2.6% +/− 0.2% SEM) calculated from n = 5 mice among 2 replicate experiments. (D) Both uninfected (panels 1 and 3) and BCG-infected CD11c+ cells (panels 2 and 4) form apparent contact with P25 T-cells in the same granuloma. Representative granulomas outlined with white dotted lines. White arrows indicate P25 T-cells, red arrows indicate BCG bacilli, green arrows indicate CD11c-eYFP cells, yellow arrows indicate contact between CD11c+ and P25 cell. The two granulomas shown in (D) were chosen as representative from an analysis of 175 total granulomas, plot shown in (E) (n = 7 mice among two replicate experiments. 25 granulomas per mouse distributed across five different 200x magnification fields). Micrographs in (D) come from 50 μm-thick tissue sections, and the entire depth of the tissue was scanned in order to verify that the CD11c+ cells identified as uninfected contained no BCG bacilli.