Figure 7. The heterotrimeric complexes exhibit differences in their ability to undergo subunit exchange.
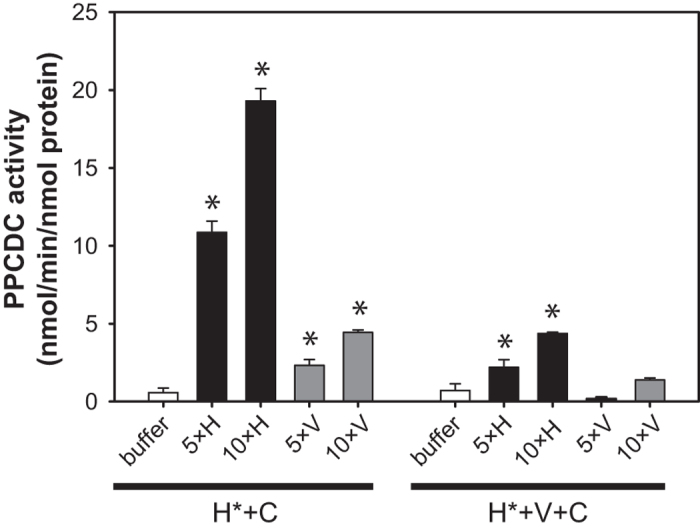
The heterotrimers Hal3PD_H378A/Cab3PD (H* + C) and Hal3PD_H378A/Vhs3PD_H459A/Cab3PD (H* + V* + C) were titrated with five- and 10-fold (relative to the monomer concentration of the inactive heterotrimer) of either Hal3PD (H) or Vhs3PD (V). The activities (reported as nmol/min/nmol protein) were adjusted to the monomer concentration of the inactive heterotrimers (i.e. excess Hal3PD or Vhs3PD added was not considered in the calculation). The data indicated the average of an experiment performed in triplicate; the error bars indicate the standard deviation. Values were compared to that of the inactive control (buffer) by one-way ANOVA followed by Dunnet’s post-test (*p < 0.05).
