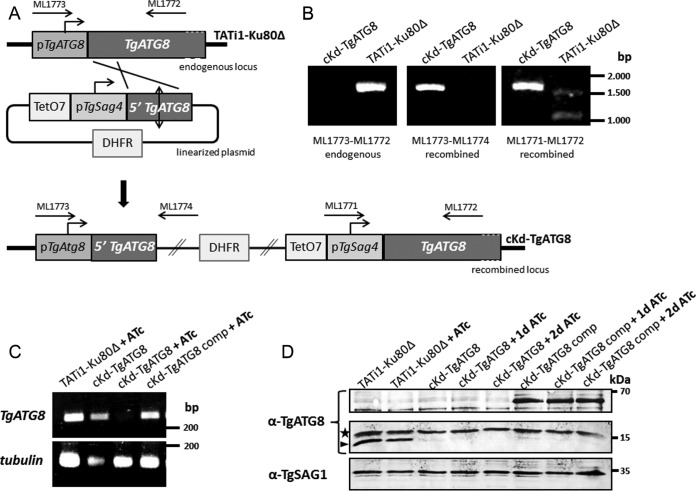
FIG 2 .
Genetic knockdown of TgATG8. (A) A TgATG8 conditional mutant cell line was generated in a parental TATi1-Ku80Δ background by replacement of the endogenous promoter with an ATc-regulated promoter. Clones were obtained after pyrimethamine selection. Arrows represent the primers used to verify integration by the PCR shown in panel B. TATi1, transactivator; TetO7, tet operator; DHFR, DHFR selection marker; pSag4, SAG4 minimal promoter. (B) Genomic DNA regions from the TATi1-Ku80Δ and cKd-TgATG8 cell lines were amplified with the primer pairs depicted in panel A for PCR assay detection of the endogenous and recombined loci. (C) Semiquantitative RT-PCR analysis of TgATG8 expression in the mutant, parental, and complemented cell lines, preceded or not by 3 days of induction with ATc to regulate expression. Specific β-tubulin primers were used as controls. (D) Western blot assay detection of TgATG8 expression in protein extracts from TATi1-Ku80Δ, cKd-TgATG8, and complemented cKd-TgATG8 parasites incubated in the absence or presence of ATc for up to 2 days. The anti-TgATG8 antibody reveals the Tomato-fused TgATG8 protein (~65 kDa) in the complemented cell line, and the endogenous TgATG8 protein (~15 kDa, arrowhead). The star designates a probable cross-reacting signal. The anti-TgSAG1 antibody was used as a loading control.