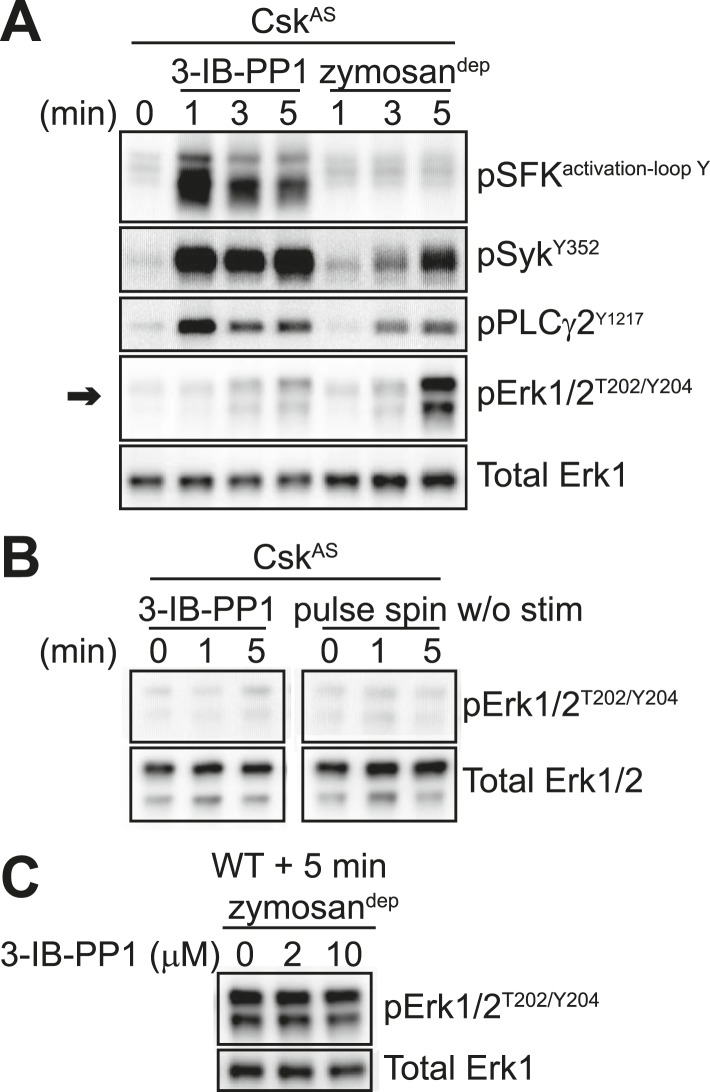
Figure 4. SFK activation in the absence of receptor clustering fails to stimulate downstream signaling in BMDMs.
(A) CskAS BMDMs were pulse-spun with 3-IB-PP1 (10 μM) or with zymosandep (10 particles per cell). Signal transduction was assessed by immunoblotting with antibodies specific to activating phosphorylation sites of the SFKs, Syk, PLCγ2, and Erk (arrow). Total Erk1 is shown as a loading control. (B) CskAS BMDMs were pulse-spun in the presence and absence of 3-IB-PP1, and Erk phosphorylation was assessed by immunoblot. Total Erk1/2 is shown as a loading control. (C) WT BMDMs were treated with zymosandep for 5 min with varying concentrations of 3-IB-PP1 and analyzed by immunoblot. See Figure 4—figure supplement 1 for additional tests of 3-IB-PP1 specificity for CskAS, Figure 4—figure supplement 2 for evidence of that 3-IB-PP1 cotreatment suppresses signaling through the CSF-1 receptor, and Figure 4—figure supplement 3 for evidence that actin-remodeling agents cannot restore 3-IB-PP1-induced downstream signaling.
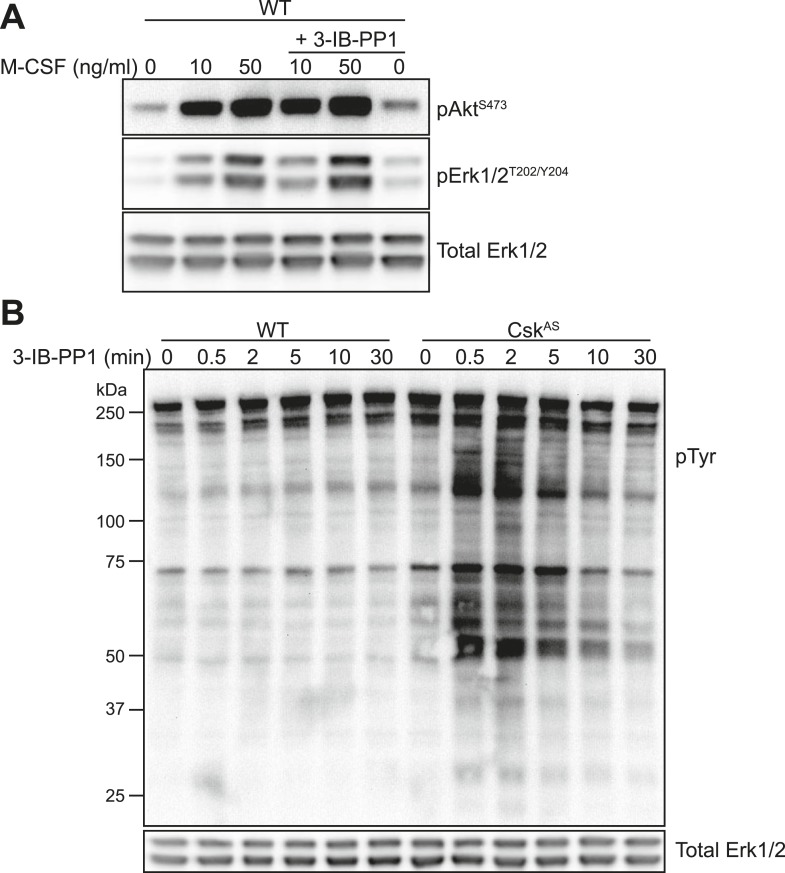
Figure 4—figure supplement 1. Further tests of 3-IB-PP1 specificity for CskAS.
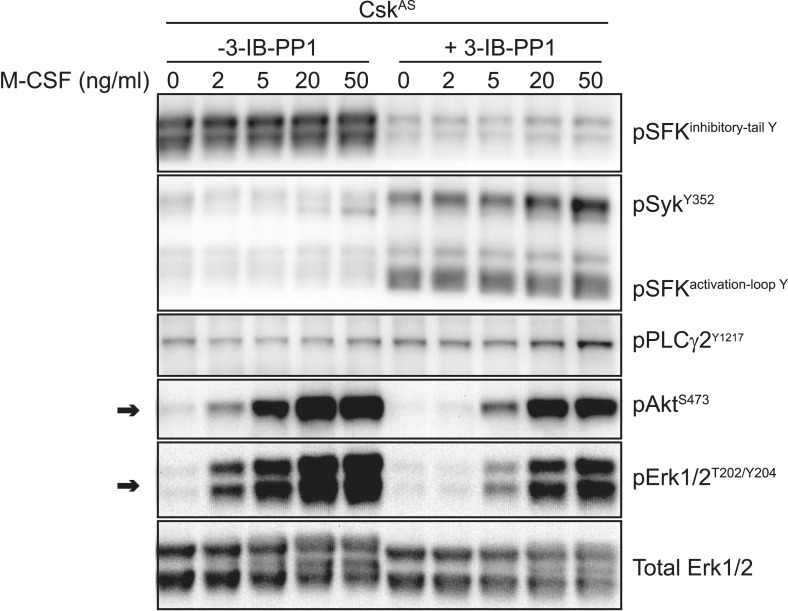
Figure 4—figure supplement 2. Inhibitory effect of 3-IB-PP1 on M-CSF-induced signaling in CskAS cells.
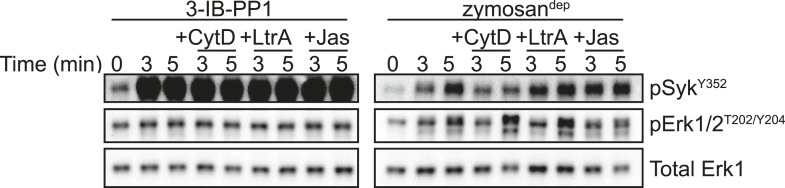
Figure 4—figure supplement 3. Actin-remodeling agents do not restore signaling downstream of 3-IB-PP1 treatment.