Abstract
Background:
Massive transfusion protocols (MTPs) are increasingly used in the transfusion practice and are developed to provide the standardized and early delivery of blood products and procoagulant agents and to supply the transfusion of blood products in a well-balanced ratio.
Aim:
The aim of this study was to investigate the effect of a hospital-wide introduction of an MTP on blood product ratio and a waste of blood products.
Materials and Methods:
Retrospective analysis was performed to compare the transfusion practice in massive bleeding patients before and after the introduction of an MTP and between the use of an MTP and transfusion off-protocol. Massive bleeding was defined as an administration of ≥5 units of red blood cells (RBCs) within 12 h.
Results:
Of 547 massively transfused patients, 192 patients were included in the pre-MTP period and 355 patients in the MTP period. The ratio of RBC to fresh frozen plasma (FFP) and the platelets transfused shifted significantly toward 1:1:1 in the MTP period (P = 0.012). This was mainly caused by a shift in RBC: FFP ratio (P = 0.014). An increase in the waste of blood products was observed, most notably FFPs (P = 0.026). Extending the storage time after thawing reduced the waste of FFPs from 11% to 4%.
Conclusion:
Hospital-wide introduction of an MTP is an adequate way to achieve a well-balanced transfusion ratio of 1:1:1. This comes at the cost of an increase in the waste of FFPs, which is lowered after extending the duration of storage time after thawing.
Keywords: Bleeding, protocol, transfusion
INTRODUCTION
Massive hemorrhage is a leading cause of mortality.[1,2,3,4] Resuscitation of massive hemorrhage has shifted toward the earlier administration of higher doses of fresh frozen plasma (FFP) to achieve hemostasis and to reduce mortality.[5,6,7,8,9,10] A balanced ratio of 1:1:1 for red blood cells (RBCs), FFPs, and platelets (PLTs) is recommended as the optimal resuscitation practice in rapidly bleeding patients.[5,10] A standardized and early delivery of blood products and procoagulant agents may contribute to achieve this optimal transfusion ratio.
Massive transfusion protocols (MTPs) are increasingly used in transfusion practice and are developed to provide standardized and early delivery of blood products and procoagulant agents,[11] to support the transfusion of higher ratios of FFPs and PLTs to RBCs[12,13] and to decrease the time to transfusion by keeping pre-thawed plasma available,[11] with the ultimate goal of improving hemorrhage control, thereby decreasing the mortality.[7,13,14,15,16,17,18,19] Previous studies investigating the MTP are focused on trauma patients. However, massive bleeding is not restricted to the trauma patients and also occurs in different patient populations, including those with gastrointestinal hemorrhage, postpartum hemorrhage, and cardiovascular surgery. Research on the effect of an MTP in nontrauma patients is limited and hampered by small sample sizes.[12,20,21] Also, besides beneficial effects, MTPs may also result in an increased waste of costly blood products, which is a major concern for blood banks.
Therefore, the aim of this observational study was to investigate the effect of the hospital-wide introduction of an MTP in massively bleeding patients on the ratio of blood products transfused and the waste of blood products.
MATERIALS AND METHODS
A retrospective analysis was performed from January 2011 to December 2013 at the Academic Medical Center, a tertiary referral center.
The MTP was introduced in January 2012. The period between January 2011 and December 2011 was identified as the pre-MTP period and the period between January 2012 and December 2013 as the MTP period. All massive bleeding patients, defined as the administration of ≥5 RBCs within 12 h, were included. This definition was chosen instead of ≥10 RBCs in 24 h, in order to capture the episodes of severe bleeding instead of oozing over a prolonged period. Also, the administration of MTP may prevent severely bleeding patients from receiving ≥10 RBCs. Of note, the patients in the MTP period who were massive bleeding and required ≥5 RBCs, but for whom the MTP was not activated, were termed as massive transfusions off-protocol. Comparisons were made between the pre-MTP period and the MTP period, and between the use of MTP and transfusion off-protocol.
Transfusion strategies
In the pre-MTP period, the patients with a suspected massive bleeding, with low hemoglobin levels (<8 g/dL) and in shock were transfused with leuco reduced RBCs, FFPs, PLTs, and procoagulant agents as deemed appropriate by the treating physician. FFPs were issued by the blood bank after the requested units were fully thawed. Recombinant factor VIIa (rVIIa) and fibrinogen were administered when a patient was still bleeding after gaining the surgical source control.
After the introduction, the MTP was activated for the patients with a systolic blood pressure <90 mmHg without the response to fluid administration and a suspicion of a massive bleeding due to any cause. Activation of the MTP provides an immediate delivery of 6 RBC units, 6 pre-thawed FFP units, and 2 PLT units (one unit is pooled from 5 donors) with the aim to administer blood products in a ratio of 1:1:1. Laboratory targets for resuscitation were a hemoglobin level of 8-10 g/dL, PT/APTT <1.5-fold extended, PLT count of 50-100 109/L, and a fibrinogen level >1.5 g/L. Additional treatment targets were normothermia (>35°C), pH level >7.25, oxygen saturation of 95% and normovolemia (base excess [BE] > −6, and a systolic blood pressure >90 mmHg). If a patient was still bleeding diffusely after gaining surgical source control while the temperature, Ph, and BE were normal, rVIIa was administered together with PLTs and fibrinogen. Six units of FFPs were kept pre-thawed for 72 h, after which they were discarded if unused. Furthermore, RBCs units and thawed plasma were discarded when not returned to the blood bank within 8 h after MTP activation; PLTs were discarded when not returned within 2 h. Blood products were also discarded after exceeding the expiration date (RBC >35 days, PLT >7 days).
Data collection
A prospectively collected blood bank transfusion database was used to determine the frequency for all products issued, as well as the number of products transfused and wasted. Patient characteristics, comorbidities, the use of anticoagulant medication and the first laboratory tests, use of procoagulant agents, length of hospital stay, length of Intensive Care Unit stay, 24-h mortality, and 28-day mortality were collected from an electronic medical record.
The primary outcome of this study was the ratio of blood products transfused (RBC: FFP:PLT). The secondary outcome was the waste of blood products.
Statistical analysis
For continuous variables, the normality assumption was tested by visually inspecting the histograms and using the Kolmogorov-Smirnov test. When the normality assumption was met, the Student's t-tests were used. Otherwise Mann-Whitney U-test was applied to test for differences between the pre-MTP and the MTP group or between the use of MTP and transfusion off-protocol. Categorical variables were compared using the Chi-square test. To determine the waste of blood products following the introduction of the MTP, a subgroup analysis was performed on all the patients for whom the MTP was activated (whether massive bleeding or not).
RESULTS
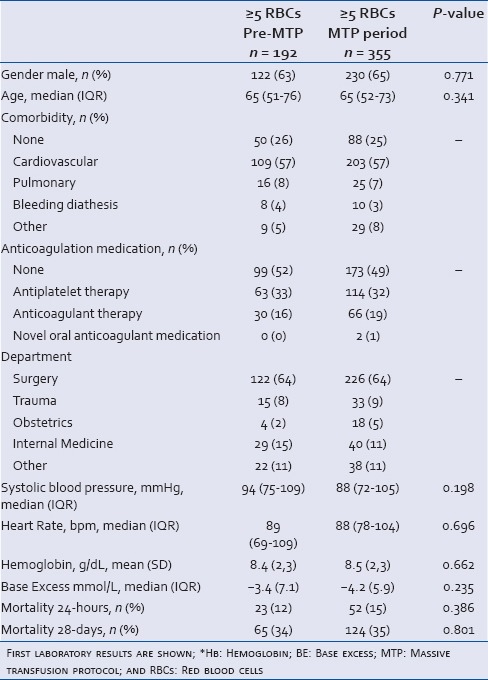
A total of 639 patients were screened, of which 547 patients met the inclusion criteria, with 192 patients in the pre-MTP period and 355 patients in the MTP period. The majority of these included patients were nontrauma patients (91%). Of note, from the total of 207 MTP activations, 56% turned out to be massively bleeding [Figure 1]. Patient characteristics and first laboratory results after bleeding are shown in Table 1. Patients before and after MTP introduction did not differ in patient characteristics.
Figure 1.
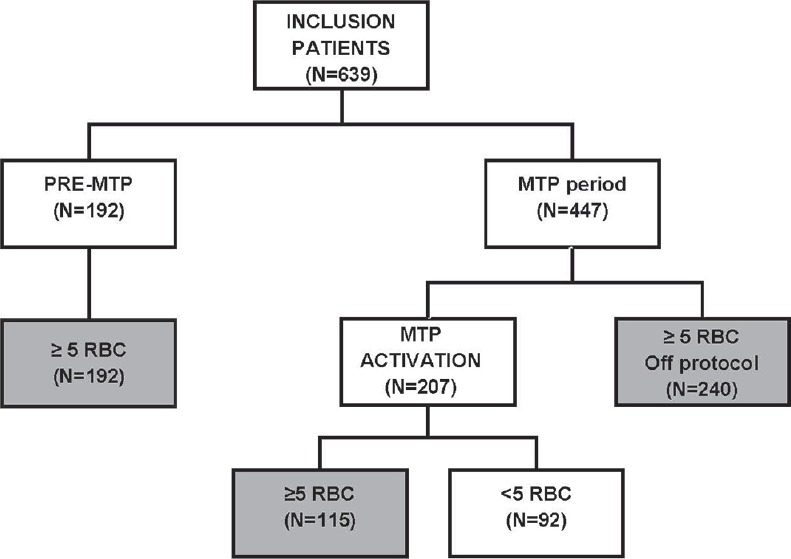
Flowchart of included patients. Only those patients who received ≥5 units of red blood cells within 12 h were included for analysis
Table 1.
Patient characteristics of massively bleeding patients (≥5 RBCs) in the pre-MTP and MTP period
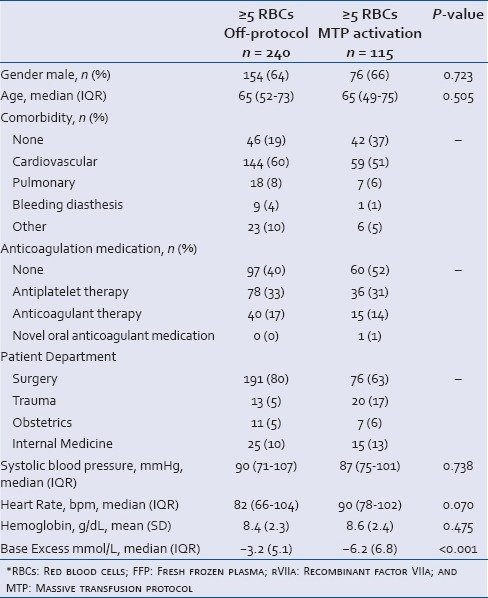
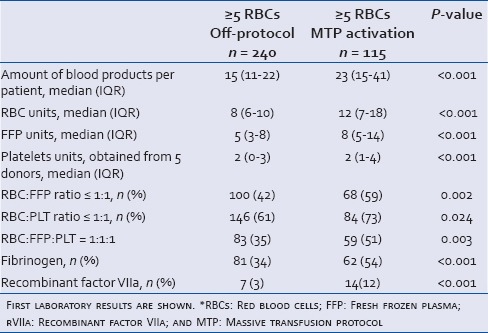
To further delineate effects of MTP, we also compared massively bleeding patients who were transfused according to the MTP with massive bleeding patients who were transfused off-protocol. Of the 447 patients in the MTP period, 240 patients received ≥5 RBCs off-protocol [Figure 1]. We were not able to retrieve the reasons why patients were transfused off-protocol. Of note, the patients for whom the MTP was activated were more acidotic compared to bleeding patients who were transfused off-protocol [Table 2].
Table 2.
Patient characteristics of massively bleeding patients (≥5 RBCs) in the MTP period. Transfusion off-protocol was compared with transfusion according to the MTP
Ratio of blood products
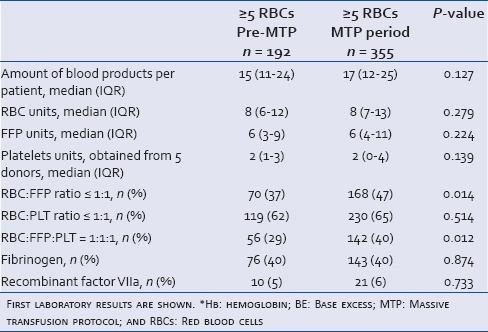
After the introduction of the MTP, the total amount of blood products administered per bleeding patient did not differ significantly compared to the pre-MTP period. Patients were significantly more frequently administered with a blood product ratio of 1:1:1 in the MTP period (P = 0.012). This was mainly caused by a shift in RBC: FFP ratio towards 1:1 (P = 0.014). No shift in the RBC: PLT ratio was observed. Massively bleeding patients in the pre-MTP group received procoagulant agents as frequently as in the MTP group [Table 3].
Table 3.
The amount of blood products and procoagulant agents transfused in massively bleeding patients, pre-MTP versus MTP period
When massively bleeding patients who were transfused according to the MTP were compared with massively bleeding patients who were transfused off-protocol, a significant increase in the total amount of blood products administered per patient was seen in patients who were transfused according to the MTP. The total amount of blood products administered per patients increased with 8 units (P < 0.001). Transfusion according to the MTP resulted in a significant shift in the transfusion ratio further towards 1:1:1 compared to transfusion off-protocol (P = 0.003). The number of FFPs and PLTs to RBCs transfused were both significantly increased. Furthermore, patients transfused according to the MTP received significantly more procoagulant agents compared to the patient's transfused off-protocol (both fibrinogen and rVIIa P < 0.001, Table 4).
Table 4.
The amount of blood products and procoagulant agents transfused in massively bleeding patients in the MTP period. Transfusion off-protocol was compared with transfusion according to the MTP
Waste of blood products
The waste of thawed FFPs increased significantly after the introduction of the MTP (P = 0.026, Figure 2a). The waste of FFPs was most frequently caused by MTP activation in the patients who turned out not to be massive bleeding (patients transfused with <5 RBCs, P = 0.02, Figure 2b). No increase in the waste of other blood products was observed.
Figure 2.
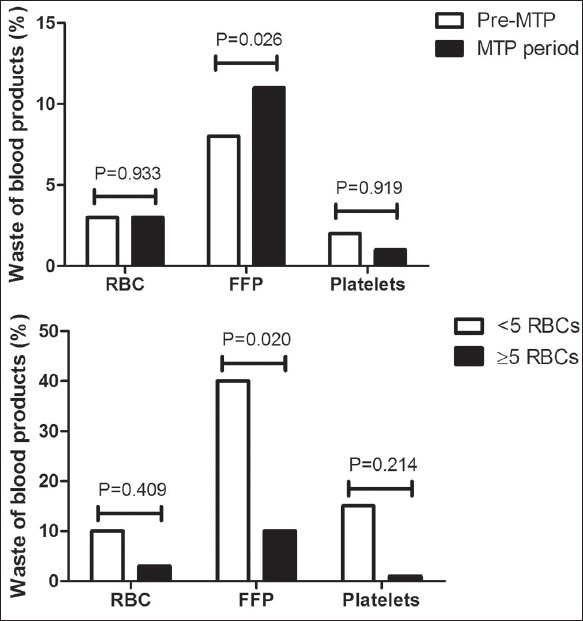
(a) The waste of blood products in the pre-massive transfusion protocol and massive transfusion protocol period. (b) The waste of blood products in the massive transfusion protocol period in patients for whom the massive transfusion protocol was activated and were massively bleeding (≥5 red blood cells) compared to patients who turned out not to be massively bleeding (<5 red blood cells). Waste was defined as the amount of blood products wasted of the total amount of transfused blood products
Furthermore, we implemented a policy of extending the storage time of thawed FFPs from 3 to 7 days for use in the MTP. This intervention led to a more than 50% reduction in the waste of pre-thawed FFPs (from 11% to 4% of all transfused FFPs) in massively bleeding patients. In all patients for whom the MTP was activated (regardless whether they were massively bleeding or not), the waste of thawed FFPs was reduced by approximately 25% (from 12% to 9%).
DISCUSSION
Use of an MTP in massively bleeding patients is associated with a shift in blood product ratio toward 1:1:1 and an increase in the waste of FFPs. Besides this shift in blood product ratio, activation of MTP in massive bleeding patients is also associated with an increase in the amount of blood products transfused and the use of procoagulant agents compared to the patients who were transfused off-protocol.
Ratio of blood products
Previous studies investigating the impact of an MTP in nontrauma patients on the amount of the transfused blood products have found either no impact[12] or a trend toward a decreased amount of RBC transfusion.[20] Furthermore, no difference was found in the ratio of blood products transfused in nontrauma patients.[12] In accordance with the results of these previous studies, we found no difference in the amount of transfused blood products. RBCs, FPPs, and PLTs were administered as frequently in the pre-MTP group as in the MTP group. Contrary to the previous studies, we found a difference in the ratio of blood products transfused. An early and standardized delivery of blood products, which was provided by the MTP, is associated with a shift in the ratio to 1:1:1. Differences in results between this study and the previous studies might be caused by differences in inclusion criteria. Administration of ≥10 units of RBCs within 24 h is a generally accepted definition for massive bleeding and is frequently used in the previous studies. However, in this study the administration of ≥5 RBCs within 12 h was defined as massive bleeding and represented the inclusion criteria. This definition was chosen instead of ≥10 RBCs in 24 h, in order to capture the episodes of severe bleeding instead of oozing over a prolonged period. Also, the administration of MTP may prevent severely bleeding patients from receiving ≥10 RBCs.
We did not find an effect on survival rate after implementation of an MTP. Data from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios trial[10] and the Prospective Observational Multicenter Major Trauma Transfusion trial[5] investigated the use of a 1:1:1 transfusion ratio in massively bleeding trauma patients and show that early administration of blood products in a 1:1:1 ratio was associated with more patients achieving hemostasis, but 24-h and 30-day mortality rate was unaffected. Death by hemorrhage occurs within the first 3 h after admission to the emergency room.[5,10] This may hamper the ability to detect a difference in mortality within 24-h or 28-day in previous studies but also in this study. Alternatively, the effect of the transfusion ratio on mortality might be less evident in nontrauma patients, which in this study the majority of patients are. Regardless the effect of the transfusion ratio on mortality, we observed in this study that introduction of the MTP is an adequate way to achieve a well-balanced transfusion ratio of blood products. This was confirmed by the comparison between patients who were transfused according to the MTP protocol and massive bleeding patients who were transfused off-protocol. Also, a significant shift was observed in RBC: FFP:PLT ratio towards 1:1:1 was observed in the MTP group. However, the comparison of patients who were transfused according to the MTP with massively bleeding patients who were transfused off-protocol is limited by a possible indication bias. The off-protocol patients might be healthier overall, which is indicated by less acidotic patients and less transfused blood products in this group. Also, a number of procoagulant agents in this group was lower. Therefore, it is likely that off-protocol patients did not have the same physiological measures that would have warranted an activation of the MTP.
Waste of blood products
In this study, we found an increase in the waste of FFPs following the introduction of an MTP as observed in previous studies in trauma patients.[12] After obtaining these results, we implemented a policy of extending the storage time of thawed FFPs from 3 to 7 days for use in the MTP. This intervention led to a more than 50% reduction in the waste of pre-thawed FFPs in massively bleeding patients and a 25% reduction in all patients for whom the MTP was activated, massive bleeding or not. Of note, an extension of the storage time from 3 to 7 days will not influence the quality of clotting factors.[22,23] The waste of FFPs was 4 times higher in the patients for whom the MTP was activated but who turned out not to be massive bleeding. Over-activation of an MTP is common and varies between 54% and 84%,[12,20,21] as specific criteria for massive bleeding in nontrauma patients are lacking. Activation criteria for the MTP are currently based on studies in trauma patients only.[24,25,26] Therefore, studies are required to identify predictors for massive bleeding in nontrauma patients. In the meantime, the feedback to the trauma team with the instructions to return products to the blood bank when not used may limit the FFP wastage.
There are several limitations to this study. It is a single center study, time to transfusion was not reported and the definition for massive bleeding patients, which we used in this study, does not control for deceased patients who were massively bleeding, but died before they could have been administered with ≥5 RBCs within 12 h. Furthermore, we have defined massive bleeding as the administration of ≥5 RBCs in 12 h instead of ≥10 RBCs in 24 h, which is a more generally accepted definition. This may hamper comparability of results.
CONCLUSION
A hospital-wide introduction of an MTP leads to a shift in blood product ratio toward a 1:1:1 in massively bleeding patients. Furthermore, the introduction of an MTP comes with an increase in a waste of FFPs, which is lowered after extending the duration of storage time after thawing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: A reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: Comprehensive population-based assessment. World J Surg. 2010;34:158–63. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 3.Ljubicic N, Budimir I, Pavic T, Bišcanin A, Puljiz Z, Bratanic A, et al. Mortality in high-risk patients with bleeding Mallory-Weiss syndrome is similar to that of peptic ulcer bleeding. Results of a prospective database study. Scand J Gastroenterol. 2014;49:458–64. doi: 10.3109/00365521.2013.846404. [DOI] [PubMed] [Google Scholar]
- 4.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 5.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: Comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutcher ME, Kornblith LZ, Narayan R, Curd V, Daley AT, Redick BJ, et al. A paradigm shift in trauma resuscitation: Evaluation of evolving massive transfusion practices. JAMA Surg. 2013;148:834–40. doi: 10.1001/jamasurg.2013.2911. [DOI] [PubMed] [Google Scholar]
- 7.Wafaisade A, Maegele M, Lefering R, Braun M, Peiniger S, Neugebauer E, et al. High plasma to red blood cell ratios are associated with lower mortality rates in patients receiving multiple transfusion (4</=red blood cell units<10) during acute trauma resuscitation. J Trauma. 2011;70:81–8. doi: 10.1097/TA.0b013e3182032e0b. [DOI] [PubMed] [Google Scholar]
- 8.Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197:565–70. doi: 10.1016/j.amjsurg.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–93. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 10.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan S, Allard S, Weaver A, Barber C, Davenport R, Brohi K. A major haemorrhage protocol improves the delivery of blood component therapy and reduces waste in trauma massive transfusion. Injury. 2013;44:587–92. doi: 10.1016/j.injury.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 12.McDaniel LM, Neal MD, Sperry JL, Alarcon LH, Forsythe RM, Triulzi D, et al. Use of a massive transfusion protocol in nontrauma patients: Activate away. J Am Coll Surg. 2013;216:1103–9. doi: 10.1016/j.jamcollsurg.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Schuster KM, Davis KA, Lui FY, Maerz LL, Kaplan LJ. The status of massive transfusion protocols in United States trauma centers: Massive transfusion or massive confusion? Transfusion. 2010;50:1545–51. doi: 10.1111/j.1537-2995.2010.02587.x. [DOI] [PubMed] [Google Scholar]
- 14.Ball CG, Dente CJ, Shaz B, Wyrzykowski AD, Nicholas JM, Kirkpatrick AW, et al. The impact of a massive transfusion protocol (1:1:1) on major hepatic injuries: Does it increase abdominal wall closure rates? Can J Surg. 2013;56:E128–34. doi: 10.1503/cjs.020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66:1616–24. doi: 10.1097/TA.0b013e3181a59ad5. [DOI] [PubMed] [Google Scholar]
- 16.O’Keeffe T, Refaai M, Tchorz K, Forestner JE, Sarode R. A massive transfusion protocol to decrease blood component use and costs. Arch Surg. 2008;143:686–90. doi: 10.1001/archsurg.143.7.686. [DOI] [PubMed] [Google Scholar]
- 17.Morse BC, Dente CJ, Hodgman EI, Shaz BH, Nicholas JM, Wyrzykowski AD, et al. The effects of protocolized use of recombinant factor VIIa within a massive transfusion protocol in a civilian level I trauma center. Am Surg. 2011;77:1043–9. [PubMed] [Google Scholar]
- 18.Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA, Jr, et al. Damage control hematology: The impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–82. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- 19.Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM, et al. Massive transfusion protocols: The role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209:198–205. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Baumann Kreuziger LM, Morton CT, Subramanian AT, Anderson CP, Dries DJ. Not only in trauma patients: Hospital-wide implementation of a massive transfusion protocol. Transfus Med. 2014;24:162–8. doi: 10.1111/tme.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morse BC, Dente CJ, Hodgman EI, Shaz BH, Winkler A, Nicholas JM, et al. Outcomes after massive transfusion in nontrauma patients in the era of damage control resuscitation. Am Surg. 2012;78:679–84. [PubMed] [Google Scholar]
- 22.Tholpady A, Monson J, Radovancevic R, Klein K, Bracey A. Analysis of prolonged storage on coagulation Factor (F)V, FVII, and FVIII in thawed plasma: Is it time to extend the expiration date beyond 5 days? Transfusion. 2013;53:645–50. doi: 10.1111/j.1537-2995.2012.03786.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Du X, Li C, Ma L, Sun P, Cao H, et al. Coagulation factors and inhibitors in thawed plasma stored at 1-6 °C for 5 days in China. Transfus Apher Sci. 2014;50:274–80. doi: 10.1016/j.transci.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: Simple as ABC (assessment of blood consumption)? J Trauma. 2009;66:346–52. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 25.Ogura T, Nakamura Y, Nakano M, Izawa Y, Nakamura M, Fujizuka K, et al. Predicting the need for massive transfusion in trauma patients: The traumatic bleeding severity score. J Trauma Acute Care Surg. 2014;76:1243–50. doi: 10.1097/TA.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 26.Olaussen A, Blackburn T, Mitra B, Fitzgerald M. Review article: Shock index for prediction of critical bleeding post-trauma: A systematic review. Emerg Med Australas. 2014;26:223–8. doi: 10.1111/1742-6723.12232. [DOI] [PubMed] [Google Scholar]


