Figure 1.
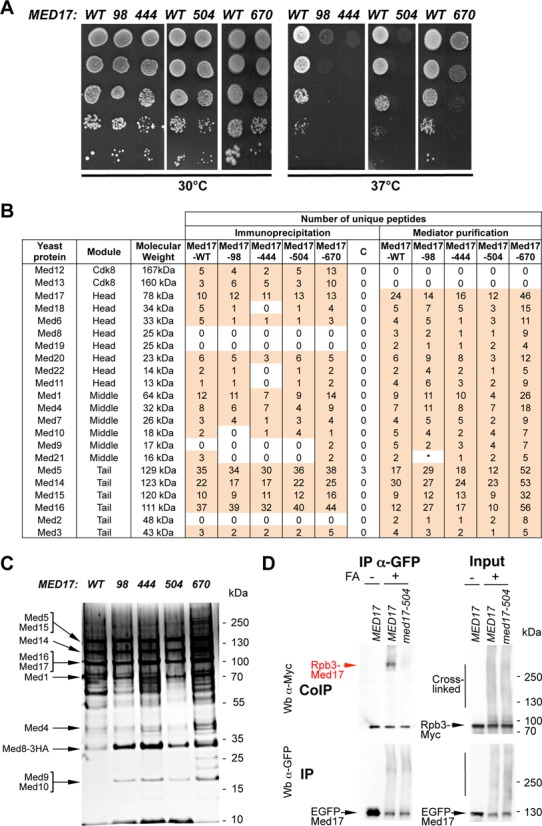
med17 thermosensitive mutants. (A) Med17 mutant phenotypes. Cultures of WT and mutant med17 yeast strains were serially diluted, spotted on YPD agar plates and incubated for 3 days at permissive (30°C) or non-permissive (37°C) temperatures. (B) Mass spectrometry analysis of Mediator integrity in med17 mutants. Mediator subunits identified by mass spectrometry analysis in WT and med17 mutants are indicated. (Immunoprecipitation) Mediator was immunoprecipitated through Med5-HA from crude extracts using magnetic protein G beads coupled to anti-HA antibodies. The MED17 strain carrying a non-tagged Mediator subunit was used as a negative control (C for Control IP). (Mediator purification) Core Mediator complex containing head, middle and tail modules was purified from med17 mutants and a wild-type strain. An asterisk marks Med21 subunit in med17-98 mutant that was detected in a second data acquisition identifying two unique peptides and also by western blotting with anti-Med21 antibody (Supplementary Figure S3B). (C) Silver-stain SDS-PAGE analysis of purified Mediator complex from the WT strain and med17 mutants. (D) Interaction between Pol II and Mediator in med17-504 mutant. Rpb3-Myc Med17-EGFP strains with WT MED17 or a med17 mutation were grown at 30°C in YPD medium and cross-linked or not with formaldehyde (FA), as indicated. Med17-EGFP was immunoprecipitated (IP) with anti-EGFP antibody from crude extracts (Input) and analyzed by western blotting with anti-Myc antibody (CoIP) against Rpb3. The cross-linked Rpb3-Med17 band is indicated in red. The position of unidentified cross-linked proteins with the tagged Med17 or Rpb3 subunits is indicated by a vertical bar.
