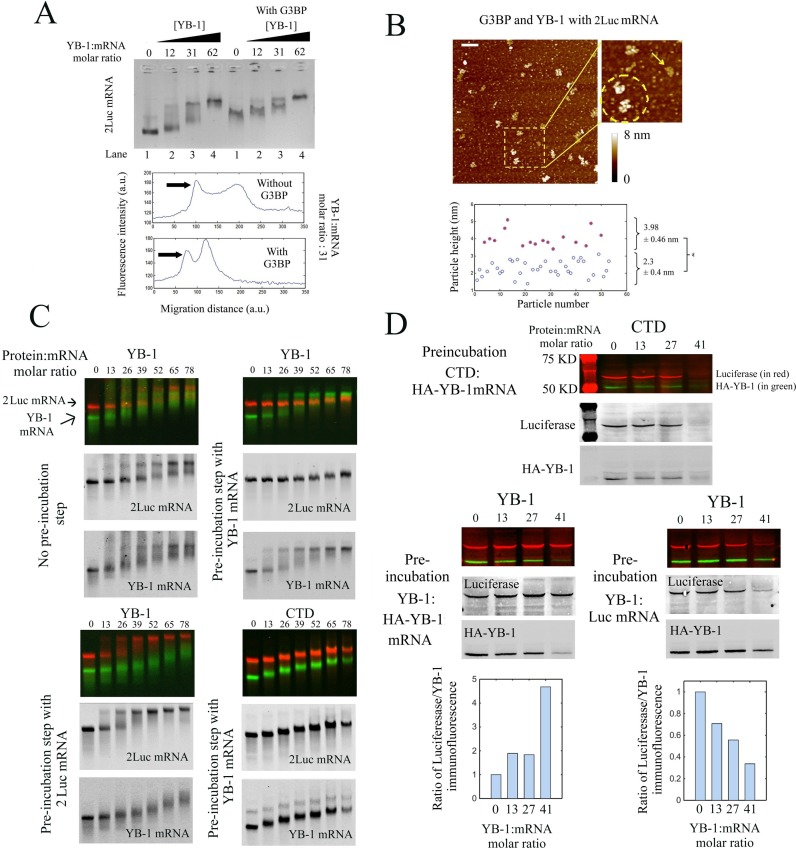
Figure 4.
The cooperative binding of YB-1 to mRNA allows a selective translational repression and takes place even in the presence of another mRNA-binding protein, G3BP. (A) Upper panel: Gel mobility shift assay probing the binding of YB-1 to 2Luc mRNA in the absence or presence of G3BP (G3BP:2 Luc mRNA molar ratio equals to 50). For a YB-1:mRNA molar ratio between 31 and 62, two bands in the gel are detected which reflects the cooperative binding of YB-1 in the presence of G3BP. Lower panel: Integrated fluorescence intensity of ethidium bromide versus the migration distance with or without G3BP extracted from the upper panel. YB-1:2Luc mRNA molar ratio: 31. In the presence of G3BP, two bands were clearly observed. (B) Higher panel: The nucleoprotein complexes formed after the interaction between 2Luc mRNA (4 nM) with an equimolar mixture of G3BP and YB-1 (50 nM each) were analyzed on mica surface by AFM. A non-homogenous binding of YB-1 to mRNA is detected on the mica surface, as shown on the higher magnification image (dashed circle: two saturated YB-1:mRNA complexes, arrow: unsaturated complex). Scale bar: 200 nm. Lower panel: Analysis of the particle heights reveals two distinct populations, which could indicated the coexistence of saturated YB-1:mRNA complexes (3.98 ± 0.46 nm) and mRNA:G3BP complexes (2.3 ± 0.4 nm). (C) Gel mobility shift assays in the presence of a mixture of fluorescently labeled 2Luc mRNA (∼3000 nt, 0.25 pmoles, red label) and YB-1 mRNA (∼1500 nt, 0.5 pmoles, green label) at varying concentrations of YB-1 or CTD. These assays were performed after mixing the two mRNAs for 15 min with or without a pre-incubation step. During the pre-incubation step, YB-1 or CTD was allowed to interact with 2Luc or YB-1 mRNA with for 15 min, as indicated in the figure panel. Both YB-1 and 2Luc mRNAs split in two distinct bands in the gel in the presence of YB-1 without the pre-incubation step, which indicates a cooperative binding of YB-1 to both mRNAs. On the other hand, when a pre-incubation step is performed, saturated YB:1mRNA complexes are solely detected for the mRNA that has been pre-incubated with YB-1, whatever it is YB-1 or 2Luc mRNA. The pre-incubation step of CTD with YB-1 mRNA failed to induce a preferential binding of YB-1 to its own mRNA. A homogenous reduction of the electrophoretic mobility for the two mRNAs is rather observed as CTD concentration increases. (D) In vitro translation assays in rabbit reticulocyte system of an equimolar mixture of Luc mRNA (∼1500 nt, 0.25 pmoles) and HA-YB-1 mRNA (∼1500 nt, 0.25 pmoles) at varying concentrations of YB-1 or CTD. HA-YB-1 or Luc mRNAs were pre-incubated for 15 min with either YB-1 or CTD, as indicated in the figure panel. The two mRNAs were then mixed and added to the reticulocyte extracts for 15 min for in vitro translation. The pre-incubation step with YB-1 leads to the preferential translation repression of the pre-incubated mRNA compared to the other mRNA. In contrast, CTD leads to an equal translation repression for both mRNAs despite the pre-incubation step. Each experiment was performed in duplicate. We also tested that the amount of protein synthesized depends on the amount of added mRNA under conditions tested. Lower panel: Plot of integrated immuno-fluorescence intensity of the bands related to luciferase and HA-YB-1 for lanes 1–6. Anti-HA and anti-luciferase antibodies were used to detect newly synthetized HA-YB-1 and luciferase, in green and red, respectively. In supplementary Figure S6B, anti-YB-1 antibody was used to control the total YB-1 concentration for the conditions tested (endogenous, exogenous and HA-YB-1).