Figure 7.
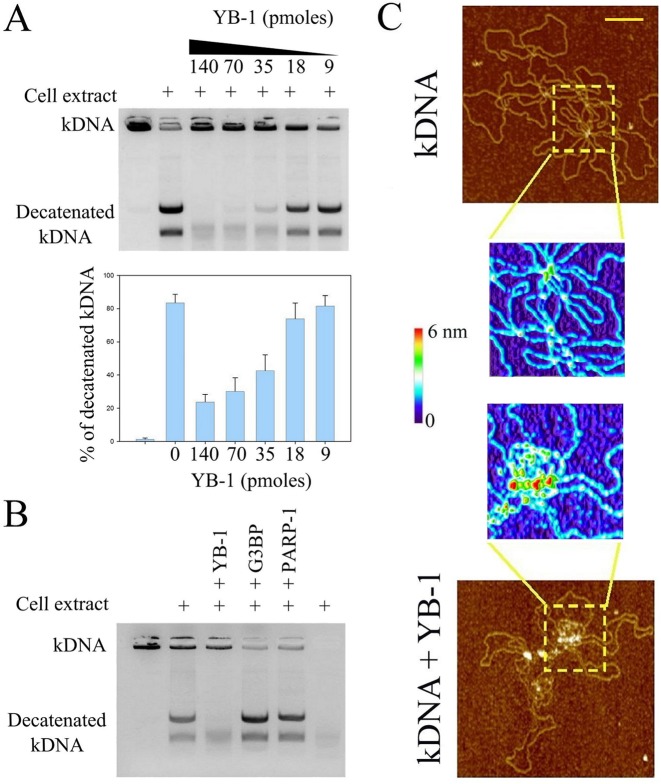
YB-1 inhibits the decatenation of kDNA mediated by topoisomerase II by masking DNA knots (A) Topoisomerase II activity from nuclear cell extract was analyzed using kinetoplast DNA (kDNA) decatenation. kDNA which contains catenated DNA circles cannot enter in the gel during electrophoresis. The results of kDNA decatenation by topoisomerase II from the cell extract (NRK cell nuclei) appear as two bands on the agarose gel. YB-1 clearly inhibits the topoisomerase II activity rather abruptly as shown in the histogram which represents the relative integrated intensity of the ethidium bromide fluorescence coming from decatenated kDNA divided by the total ethidium bromide fluorescence of kDNA (catenated plus decatenated kDNA). kDNA: 300 ng per well. Results are mean ± SD over three experiments. (B) Topoisomerase II activity from nuclear cell extract in the absence or presence of YB-1, G3BP or PARP-1. Only YB-1 induces a significant inhibition of the topoisomerase II activity. Protein amount: 35 pmoles per well. kDNA: 300 ng per well. (C) kDNA was observed on mica by AFM in the absence or presence of YB-1 (1 μM). The higher magnification images show that YB-1 is located at DNA knots. kDNA: 2 μg/ml. Scale bar: 200 nm.