Figure 5. Structural basis of 67LR inhibition by NSC47924.
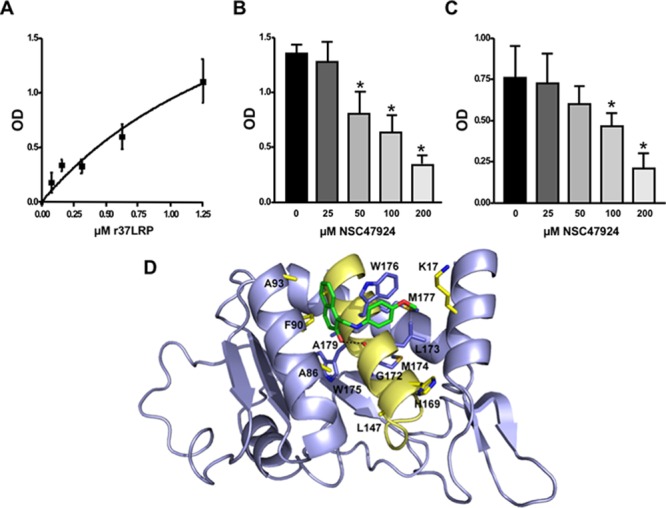
A. Increasing concentrations of purified human His-tagged recombinant 37LRP (r37LRP) were placed for 1 hour at 37°C on wells coated with 1 μg of LM. Bound r37LRP was revealed by anti-His-HRP and OPD staining; the absorbance at 490 nm was measured. r37LRP binding to BSA-coated wells was subtracted to obtain specific binding. Values represent the mean ± SD of three experiments carried out in triplicate; (*, P < 0.05), as determined by the Student's t test. Human r37LRP binds to LM in a dose dependent manner. B. r37LRP was placed for 1 hour at 37°C on LM-coated wells in the presence of increasing concentrations of NSC47924 or DMSO (■), as a vehicle control. Bound r37LRP was revealed by anti-His-HRP and OPD staining; the absorbance at 490 nm was measured. r37LRP binding to BSA-coated wells was subtracted to obtain specific binding. Values represent the mean ± SD of three experiments carried out in triplicate; (*, P < 0.05), as determined by the Student's t test. C. r37LRP was placed for 1 hour at 37°C on wells coated with 100 μg of YIGSR in the presence of increasing concentrations of NSC47924 or DMSO (■), as a vehicle control. Bound r37LRP was revealed by anti-His-HRP and OPD staining; the absorbance at 490 nm was measured. r37LRP binding to BSA-coated wells was subtracted to obtain specific binding. Values represent the mean ± SD of three experiments carried out in triplicate; (*, P < 0.05), as determined by the Student's t test. D. Predicted binding mode of NSC47924. The most populated and lowest energy pose is shown for NSC47924 docked into the 37LRP crystal structure (slate blue cartoon). NSC47924 is shown in stick, with carbons in green, nitrogens in blue, and oxygen in red. H-bonds interactions are shown with dashed black lines. The protein residues are shown in yellow stick, while the palindromic key residues are displayed in blue sticks. Human recombinant 37LRP binds LM and is inhibited by NSC47924; NSC47924 engages many contacts with residues of 37LRP peptide G.
