Figure 1. Overview of PDAC cell metabolism in response to microenvironment constraints and oncogenic signals.
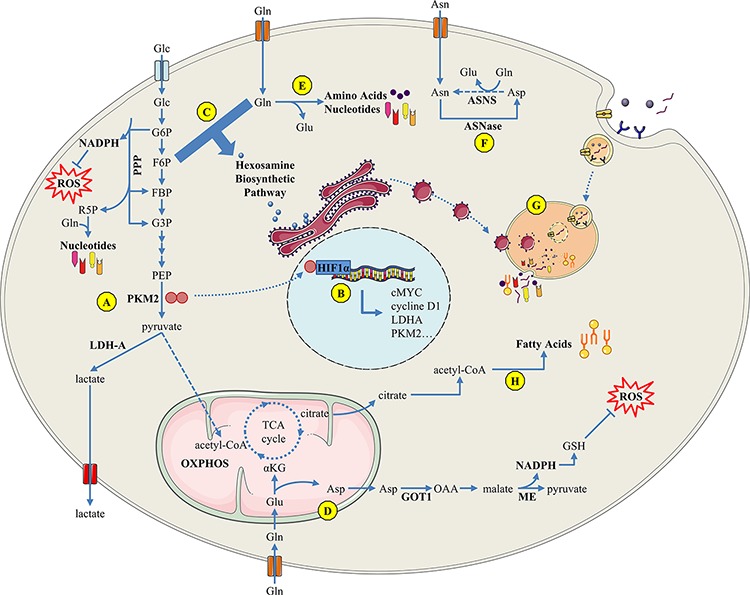
A. The Warburg effect sustains metabolic needs of PDAC proliferative cells; B. The PKM2 tyrosine kinase enhances transcriptional activity of several factors such as hypoxia-inducible factor HIF1-α, inducing the Warburg effect through a positive feedback loop; C. the hexosamine biosynthetic pathway uses glucose and glutamine influx for protein O-GlcNAc glycosylation and its inhibition induces an unfolded-protein response-dependent cell death; D. PDAC-specific glutamine metabolism: glutamine-derived aspartate is converted into oxaloacetate, then into malate, and finally into pyruvate, resulting in an increased NADPH/NADP+ ratio that provides the reducing power to maintain reduced glutathione pools; E. glutamine is a nitrogen donor for amino acid and nucleotide biosynthesis; F. ASNase may be a promising therapy since a majority of PDAC express no or low ASNS; G. macropinocytosis and autophagy support the metabolic needs of PDAC cells; H. PDAC overexpresses enzymes involved in fatty acid synthesis. Glc : glucose; Gln: glutamine; Glu : glutamate; Asn : asparagine; ASNase : asparaginase; ASNS : asparagine synthetase; GSH : glutathion; LDH-A : lactate dehydrogenase-A; ME : malic enzyme; NADP : nicotinamide adenine dinucleotide phosphate; OXPHOS : oxidative phosphorylation; PKM : pyruvate kinase muscle-isozyme.
