Abstract
Aims/Introduction
Greater glycemic variability and lack of predictability are important issues for patients with type 1 diabetes. Dietary factors are one of the contributors to this variability, but how closely diet is linked to glycemic fluctuation on a daily basis has not been investigated. We examined the association between carbohydrate intake and glycemic excursion in outpatients.
Materials and Methods
A total of 33 patients with type 1 diabetes were included in the analyses (age 44.5 ± 14.7 years, diabetes duration 15.1 ± 8.3 years, 64% female, 30% using insulin pump, glycated hemoglobin 8.1 ± 1.3%). Time spent in euglycemia (70–180 mg/dL), hyperglycemia (>180 mg/dL) and hypoglycemia (<70 mg/dL) of consecutive 48-h periods of continuous glucose monitoring data were collected together with simultaneous records of dietary intake, insulin dose and physical activity. Correlation analyses and multiple regression analyses were used to evaluate the contribution of carbohydrate intake to time spent in the target glycemic range.
Results
In multiple regression analyses, carbohydrate intake (β = 0.53, P = 0.001), basal insulin dose per kg per day (β = −0.31, P = 0.034) and diabetes duration (β = 0.30, P = 0.042) were independent predictors of time spent in euglycemia. Carbohydrate intake (β = −0.51, P = 0.001) and insulin pump use (β = −0.34, P = 0.024) were independent predictors of time spent in hyperglycemia. Insulin pump use (β = 0.52, P < 0.001) and bolus insulin dose per kg per day (β = 0.46, P = 0.001) were independent predictors of time spent in hypoglycemia.
Conclusions
Carbohydrate intake is associated with time spent in euglycemia in patients with type 1 diabetes.
Keywords: Dietary carbohydrates, Glycemic control, Type 1 diabetes mellitus
Introduction
Glucose variability is greater in type 1 diabetes patients than it is in type 2 diabetes patients1–3, and the lack of predictability is an important issue for type 1 diabetes patients and medical staff in daily practice. Hypoglycemia is a complication of diabetes treatment, and the frequency of severe hypoglycemia increases when glucose is lowered. Larger glucose variability is an additional risk factor for severe hypoglycemia4. Minimizing glucose variability is therefore a plausible method for offsetting the increased risk of hypoglycemia associated with tight glycemic control. In contrast, type 1 diabetes patients with larger glycemic variability retain higher glycated hemoglobin (HbA1c), partly because of the difficulty in raising the insulin dosage or physical activity due to fear of hypoglycemia4,5. Larger glucose variability is associated with lower quality of life and lower satisfaction of treatment6.
Clinical factors contributing to hypoglycemia and glycemic variability have been investigated, and can be summarized as dietary factors, physical activity, insulin regimen, monitoring and pathophysiological conditions7–11. There have been few studies of dietary factors; however, Maahs et al.9 reported a positive association between carbohydrate intake and glucose excursion during 1–4 h after the first meal of the day. In contrast, lower carbohydrate intake was associated with higher HbA1c in the Diabetes Control and Complications Trial12. Further research is required to learn the details of the association of carbohydrate intake with glycemic control and glycemic variability on a daily basis.
Recently, continuous blood glucose monitoring (CGM) has made it possible to monitor in detail glucose fluctuations in daily life, and several indicators of intraday and interday glycemic variability are now available4. Time spent in the glycemic target range is a simple and absolute assessment of glycemic control, reflecting both mean glucose level and glucose excursions, and is sensitive to interventions13.
To investigate the overall influence of carbohydrate intake on glycemic levels including both excursion and mean, we evaluated the association between carbohydrate intake and time spent in the target glycemic range by use of simultaneous dietary records and CGM in outpatient settings.
Materials and Methods
Participants
The present cross-sectional study was carried out in Kyoto University Hospital between September 2011 and June 2012. Patients aged 18 years or older who were diagnosed with type 1 diabetes and were treated with basal–bolus therapy (multiple daily injection [MDI] or continuous subcutaneous insulin infusion [CSII]) were eligible for enrolment. Patients were excluded if they had renal insufficiency (creatinine ≥1.5 mg/dL), liver failure, acute infection, psychological comorbidities or dementia, were pregnant, taking steroid medication, or had received pancreas or islet transplantation. The study protocol was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee, and registered on University hospital Medical Information Network in Japan (UMIN000005833). Written informed consent was obtained from all participants.
Continuous Glucose Monitoring
All participants underwent a 72-h period of monitoring using CGMS® system Gold™ (Medtronic, Northridge, CA, USA). The CGMS® consists of a glucose oxidase-based sensor inserted subcutaneously in an abdominal site and attached through cable to a monitor. The monitor takes a reading every 10 s and accumulates an average every 5 min for a total of 288 readings per day. The participants were instructed to enter at least four daily metered blood glucose measurements for calibration using conventional glucose meters. They also kept records of insulin dose and whether or not they carried out bolus adjustment by carbohydrate-counting while wearing CGMS®. The CGM data could be read only after CGMS® was removed. The data from CGMS® were downloaded using Minimed solutions CGMS sensor 3.0C software. Further data management was carried out using Microsoft Excel (Microsoft, Seattle, WA, USA). According to Medtronic's recommendation, the criteria that a minimal correlation of 0.79 between the sensor glucose and meter blood glucose values with a mean absolute error <28% were used to determine the accuracy of the CGM data. Patients whose CGM data deviated from these criteria were rescheduled for wearing CGMS® with a dietary record and physical activity monitoring, and this second set of CGM data was used for the analyses so long as the participants met the criteria. Glucose values outside the range of 40–400 mg/dL were reported as ≤40 or ≥400 mg/dL. The first complete, consecutive 48-h period of valid CGM data beginning from midnight was analyzed for each subject.
Glycemic Indices
Mean, standard deviation (SD) and time spent in euglycemia (70–180 mg/dL), in hyperglycemia (>180 mg/dL) and in hypoglycemia (<70 mg/dL) were calculated from CGM data. Time spent in the target range, expressed as a percentage in a total 48-h monitoring period, has been used in several studies for assessing CGM data13,14. Time spent in euglycemia is a direct index of appropriate glycemic control, and can reflect both mean glucose and glucose excursions13.
Dietary Data
Patients recorded everything they ate for the 72-h period using recording sheets and photographs of foods. We used two, complete, consecutive whole-day records from 0.00 to 24.00 h. The photographs were taken before and after consumption with a ruler placed beside the foods as a scale for estimation of the size of the portions. The validity of assessing diet using photographs as food intake records has been reported15. Registered dietitians estimated the amount of foods from the recording sheets and photographs, and calculated the weight of the ingredients, carbohydrate, fat, protein, fiber and ethanol using computer software program Excel Eiyo-kun version 4.5 (Kenpaku Co. Ltd, Tokyo, Japan). Excel Eiyo-kun is a program designed to calculate amounts of ingredients based on standard tables of food composition in Japan16,17.
The composition of fat (% of energy) and protein (% of energy) were calculated as follows: fat (g) × 9/total energy (kcal) and protein (g) × 4/total energy (kcal), respectively. The composition of carbohydrate (% of energy) was calculated as 100–(fat [% of energy] + protein [% of energy]). These calculations were used to determine fat intake, protein intake and carbohydrate intake in the following analyses.
We defined snacking after dinner as a small intake of food and/or beverage with energy after dinner excepting those for treating hypoglycemia, and ‘Snacking after dinner’ was coded 1 for any episode of snacking after dinner during the 2 days, and 0 otherwise. ‘Late dinner’ was coded 1 for any dinner after 21.00 h during the 2 days, and 0 otherwise.
Physical Activity Level
We measured the physical activity using Lifecoder PLUS® (Suzuken, Nagoya, Japan) during CGM. This method involves uniaxial accelerometry and has been validated for assessment of physical activity-related energy expenditure18. The patients attach it onto a belt when they awake and take it off before they go to sleep. The device samples acceleration at 32 Hz and assesses values ranging from 0.06 to 1.94 g (1.00 g is equal to the acceleration of free fall). A maximum pulse over 4 s is taken for the acceleration value, and activity is categorized into 11 levels. The activity levels are subsequently converted to calculate energy expenditure due to various activities (kcal). Total energy expenditure (TEE) is calculated from the sum of the basal metabolic rate, thermic effect of food (=1/10[TEE]) and energy expenditure as a result of activity. Basal metabolic rate is calculated from bodyweight, height, sex and age using a standard Japanese formula19. The physical activity level is calculated as follows: TEE (kcal)/basal metabolic rate (kcal). The average of two consecutive days that coincided with the 2 days of CGM data was used.
Coefficient of Variation of R-R Intervals
We measured the coefficient of variation of R-R intervals (CVR-R) for assessment of autonomic neuropathy20,21. The R-R interval was measured by electrocardiography for 1 min in the supine position after at least 3 min rest using CardioStar FCP-7301 (Fukuda Denshi, Tokyo, Japan). The CVR-R was calculated by dividing the SD by the mean (M): CV (%) = (SD/M) × 100.
Laboratory Measurements
Glycemic control was assessed by HbA1c and glycated albumin (GA). In all patients, HbA1c and GA levels were evaluated within 2 weeks after CGM was initiated. HbA1c was measured by reversed-phase cation exchange chromatography, using ADAMS™A1c HA-8180 (Arkray, Kyoto, Japan). The CV of within-run reproducibility and between-run reproducibility were reported to be within 1%. The value for HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program equivalent value (%) calculated by the formula HbA1c (%) = 1.02 × HbA1c Japan Diabetes Society (%) + 0.25%, measured by the previous Japanese standard substance and measurement methods and HbA1c (National Glycohemoglobin Standardization Program)22. GA was measured by the enzymatic method, using JCA-BM8000 series (JOEL, Tokyo, Japan). The CV of within-run reproducibility is 0.8–1.0%, and that of between-run reproducibility is 1.2%. Serum C-peptide was measured by Chemiluminescent Enzyme Immunoassay, LUMIPULSE® Presto C-peptide (Fuji Rebio, Tokyo, Japan). The CV of within-run reproducibility is 1.96–2.97%, and that of between-run reproducibility is 1.06–2.60%. The measurement range of serum C-peptide is 0.02–30 ng/mL. For C-peptide measurement, blood samples were centrifuged immediately at 1,880 g for 5 min. Serum was stored at −80°C and measured within 1 month after collection.
Glucagon Stimulation Test
A glucagon stimulation test was carried out after overnight 8-h fast. Tests were rescheduled if the participant had a capillary glucose value <70 mg/dL. Serum C-peptide was measured before and 6 min after the intravenous injection of 1 mg glucagon23.
Hypoglycemia
The participants were asked about their experience of hypoglycemia over the past year on the day of starting CGM, and a Clarke score (0 = no hypoglycemia; ≥4 = hypoglycemia unawareness) was calculated24.
Statistical Analyses
Analysis was carried out using STATA® 11.2 (StataCorp LP, College Station, TX, USA). Pearson's product–moment correlation coefficient was used to evaluate the relationship between glycemic indices and clinical variables including dietary intake. We then carried out multiple regression analyses for the association between carbohydrate intake and glycemic indices adjusting for other plausible determinants of glycemic indices. We started from an empty model and added variables by significance level <0.10 from the following candidate variables: age, diabetes duration, body mass index (BMI), blood pressure, CSII use, carbohydrate counting, fiber intake, snacking after dinner episode, late dinner, insulin dose per kg per day, physical activity, CVR-R, lipid profiles, C-peptide and Clarke score in the order of correlation coefficients with glycemic indices. Two-sided P < 0.05 was considered statistically significant.
Results
A total of 40 patients were recruited. Diet therapy for these patients was based on advice from nutritionists. Of the 40 patients, one was found to be in a non-insulin-dependent-state by serum C-peptide level25. In three patients, available CGM data covered <48 h because of disconnection of the sensor or calibration errors. One patient failed to measure CVR-R because of atrial fibrillation. In another patient, there were missing data because of problems with Lifecoder® PLUS. Another patient reported that he drank a large amount of alcohol (equivalent to more than 60 g of pure ethanol per day) during monitoring because of a celebration party. These seven patients were excluded from the analysis. None of the 33 remaining patients had clinical thyroid disease or were diagnosed with clinical ketosis.
The clinical characteristics and the measurements of the 33 patients analyzed are shown in Table1. The mean age was 44.5 years, and most of them were not obese. Their C-peptide levels were very low, and most were undetectable by highly sensitive measurement. The distribution of intake of the three macronutrients is shown in Figure1. Carbohydrate intake correlated negatively with fat intake (r = −0.93, P < 0.001), although there was no significant correlation between carbohydrate intake and protein intake (r = −0.33, P = 0.061) or between protein intake and fat intake (r = −0.04, P = 0.819).
Table 1.
Clinical characteristics of participants
| Age (years) | 44.5 ± 14.7 |
| Sex | |
| Male | 12 (36) |
| Female | 21 (64) |
| BMI (kg/m2) | 22.6 ± 3.6 |
| Diabetes duration (years) | 15.1 ± 8.3 |
| Glucagon stimulated C-peptide | |
| Detectable (≥0.02 ng/mL) | 5 (15) |
| Undetectable | 28 (85) |
| Fasting blood glucose (mg/dL) | 188 ± 66 |
| Insulin administration | |
| MDI | 23 (70) |
| CSII | 10 (30) |
| Carbohydrate counting | 7 (21) |
| HbA1c (%) | 8.1 ± 1.3 |
| Glycated albumin (%) | 24.2 ± 4.8 |
| Lipid profile (n = 32) | |
| LDL cholesterol (mg/dL) | 97 ± 24 |
| HDL cholesterol (mg/dL) | 74 ± 18 |
| Triglyceride (mg/dL) | 86 ± 56 |
| Clarke score | 1.6 ± 1.3 |
| CVR-R (%) | 3.3 ± 1.8 |
| Physical activity | 1.4 ± 0.1 |
| Blood pressure (mmHg) | |
| Systolic | 119 ± 17 |
| Diastolic | 69 ± 10 |
| Total insulin dose/BW (U/kg) | 0.69 ± 0.21 |
| Basal insulin dose/BW (U/kg) | 0.29 ± 0.10 |
| Bolus insulin dose/BW (U/kg) | 0.40 ± 0.16 |
| Glycemic indices measured by CGM | |
| Mean glucose (mg/dL) | 160 ± 48 |
| SD (mg/dL) | 64 ± 16 |
| Time spent in euglycemia (70–180 mg/dL) during 48 h (min) | 1520 ± 566 |
| (%) | 52.8 ± 19.6 |
| Time spent in hyperglycemia (>180 mg/dL) during 48 h (min) | 1018 ± 692 |
| (%) | 35.3 ± 24.0 |
| Time spent in hypoglycemia (<70 mg/dL) during 48 h (min) | 342 ± 403 |
| (%) | 11.9 ± 14.0 |
| Dietary data (average of consecutive 2 days) | |
| Energy, including alcohol (kcal) | 1922 ± 400 |
| Energy (including alcohol)/BW (kcal/kg) | 33.0 ± 5.3 |
| Energy (kcal) | 1888 ± 370 |
| Energy/BW (kcal/kg) | 32.4 ± 5.0 |
| Carbohydrate intake (% of energy) | 52.2 ± 5.5 |
| Fat intake (% of energy) | 32.7 ± 5.2 |
| Protein intake (% of energy) | 15.1 ± 2.0 |
| Fiber (g) | 12.6 ± 3.6 |
| Ethanol (g) | 3.6 ± 7.4 |
| Late dinner (%) | 7 (21) |
| Snacking after dinner (%) | 15 (45) |
Data are mean ± standard deviation (SD) or n (%). BMI, body mass index; BW, bodyweight; CGM, continuous glucose monitoring; CVR-R, coefficient of variation of R-R intervals; Energy (including alcohol), total energy intake including alcohol; Energy (including alcohol)/BW, total energy intake including alcohol per bodyweight; Energy, total energy intake excluding alcohol; Energy/BW, total energy intake excluding alcohol per bodyweight; CSII, continuous subcutaneous insulin infusion; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDI, multiple daily injection.
Figure 1.
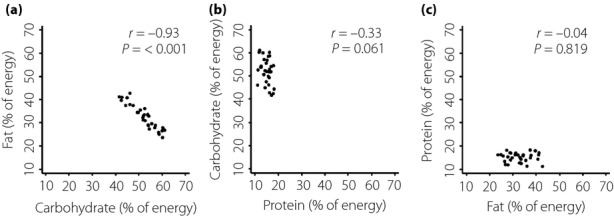
Distribution of intake of three macronutrients (% of energy) shown by two-way scatter plot. (a) Fat intake and carbohydrate intake. (b) Carbohydrate intake and protein intake. (c) Protein intake and fat intake.
Carbohydrate intake correlated positively with time spent in euglycemia (r = 0.48, P = 0.005) and negatively with time spent in hyperglycemia (r = −0.50, P = 0.003; Figure2). Conversely, fat intake showed a negative correlation with time spent in euglycemia (r = −0.44, P = 0.011) and a positive correlation with time spent in hyperglycemia (r = 0.45, P = 0.009). Neither carbohydrate intake nor fat intake correlated with time spent in hypoglycemia (r = 0.20, P = 0.274 and r = −0.15, P = 0.400, respectively). In addition, there was no significant relationship between protein intake and time spent in euglycemia (r = −0.17, P = 0.331), hyperglycemia (r = 0.23, P = 0.206) or hypoglycemia (r = −0.14, P = 0.425).
Figure 2.

(a) Correlations between carbohydrate intake and time spent in euglycemia, hyperglycemia and hypoglycemia. (b) Correlations between fat intake and time spent in euglycemia, hyperglycemia and hypoglycemia.
The relationships between insulin dose and time spent in euglycemia, hyperglycemia and hypoglycemia are shown in Figure3. Total insulin dose per kg per day was positively correlated with time spent in hypoglycemia (r = 0.47, P = 0.006), but not with time spent in euglycemia (r = −0.15, P = 0.409) or hyperglycemia (r = −0.15, P = 0.400). Basal insulin dose per kg per day was not correlated with time spent in euglycemia (r = −0.27, P = 0.135), hyperglycemia (r = 0.11, P = 0.543) or hypoglycemia (r = 0.18, P = 0.303). Bolus insulin dose per kg per day showed a positive correlation with time spent in hypoglycemia (r = 0.48, P = 0.005), but not with time spent in euglycemia (r = −0.03, P = 0.879) or hyperglycemia (r = −0.25, P = 0.152).
Figure 3.

(a) Correlations between total insulin dose and time spent in euglycemia, hyperglycemia and hypoglycemia. (b) Correlations between basal insulin dose and time spent in euglycemia, hyperglycemia and hypoglycemia. (c) Correlations between bolus insulin dose and time spent in euglycemia, hyperglycemia and hypoglycemia. BW, bodyweight.
We carried out multiple regression analyses for time spent in euglycemia, hyperglycemia and hypoglycemia by forward selection from possible determinants considering simple correlation coefficients. Independent variables for time spent in euglycemia were selected from carbohydrate intake, basal insulin dose per kg per day, diabetes duration, carbohydrate counting, fiber, triglyceride, snacking after dinner, systolic blood pressure, physical activity, CVR-R, BMI, CSII use, Clarke score, late dinner, age and glucagon-stimulated C-peptide in this order. Independent variables for time spent in hyperglycemia were selected from carbohydrate intake, CSII use, CVR-R, bolus insulin dose per kg per day, age, carbohydrate counting, triglyceride, late dinner, diabetes duration, snacking after dinner, glucagon-stimulated C-peptide, BMI, fiber, systolic blood pressure, Clarke score and physical activity, in this order. Independent variables for time spent in hypoglycemia were selected from CSII use, bolus insulin dose per kg per day, fiber, age, CVR-R, late dinner, carbohydrate intake, BMI, physical activity, systolic blood pressure, glucagon-stimulated C-peptide, snacking after dinner, diabetes duration, triglyceride, carbohydrate counting and Clarke score, in this order. We found that time spent in euglycemia was significantly predicted by carbohydrate intake (β = 0.53, P = 0.001), basal insulin dose per kg per day (β = −0.31, P = 0.034) and diabetes duration (β = 0.30, P = 0.042), and that time spent in hyperglycemia was predicted by carbohydrate intake (β = −0.51, P = 0.001) and CSII use (β = −0.34, P = 0.024; Table2). Time spent in hypoglycemia was predicted by CSII use (β = 0.52, P < 0.001) and bolus insulin dose per kg per day (β = 0.46, P = 0.001).
Table 2.
Multivariate analyses for the determinants of time spent in target range
| Covariates | Time spent in euglycemia (70–180 mg/dL) | Time spent in hyperglycemia (>180 mg/dL) | Time spent in hypoglycemia (<70 mg/dL) | |||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| Carbohydrate intake | 0.53 | 0.001 | −0.51 | 0.001 | – | |
| Basal insulin dose/BW | −0.31 | 0.034 | – | – | ||
| Diabetes duration | 0.30 | 0.042 | – | – | ||
| Carbohydrate counting | 0.26 | 0.078 | – | – | ||
| CSII | – | −0.34 | 0.024 | 0.52 | <0.001 | |
| Bolus insulin dose/BW | – | – | 0.46 | 0.001 | ||
| Late dinner | – | – | 0.24 | 0.065 | ||
| Adjusted R2 | 0.41 | 0.33 | 0.50 | |||
| P | <0.001 | <0.001 | <0.001 | |||
Variables were selected by the forward selection method, according to the significance level for addition of variables <0.10, from the following candidate variables: carbohydrate intake, insulin dose per kg per day, diabetes duration, carbohydrate counting, continuous subcutaneous insulin infusion (CSII) use, late dinner, fiber intake, lipid profiles, snacking after dinner episode, blood pressure, physical activity, coefficient of variation of R-R intervals (CVR-R), Clarke score, body mass index, age and C-peptide. Basal insulin dose/BW, basal insulin dose per bodyweight; Bolus insulin dose/BW, bolus insulin dose per bodyweight.
We also analyzed the relationship between carbohydrate intake and HbA1c or GA as indicators of average glycemia over several months or weeks, respectively, and the relationship between carbohydrate intake and glycemic indices calculated from CGM data (Figure4). There was no significant correlation between carbohydrate intake and HbA1c (r = −0.14, P = 0.438) or GA (r = −0.10, P = 0.590). In contrast, carbohydrate intake was significantly correlated with mean glucose calculated from CGM data (r = −0.48, P = 0.005), SD (r = −0.37, P = 0.033) calculated from CGM data and also time spent in euglycemia (r = 0.48, P = 0.005) calculated from CGM data. The strongest correlation was observed between HbA1c and GA (r = 0.85, P < 0.001), and a relatively strong correlation was observed between HbA1c and mean, between GA and mean, and between mean and time spent in euglycemia (r = 0.70, r = 0.65 and r = −0.69, respectively, all P < 0.001). Time spent in euglycemia was moderately correlated with HbA1c (r = −0.45, P = 0.008), but not with GA (r = −0.31, P = 0.083).
Figure 4.
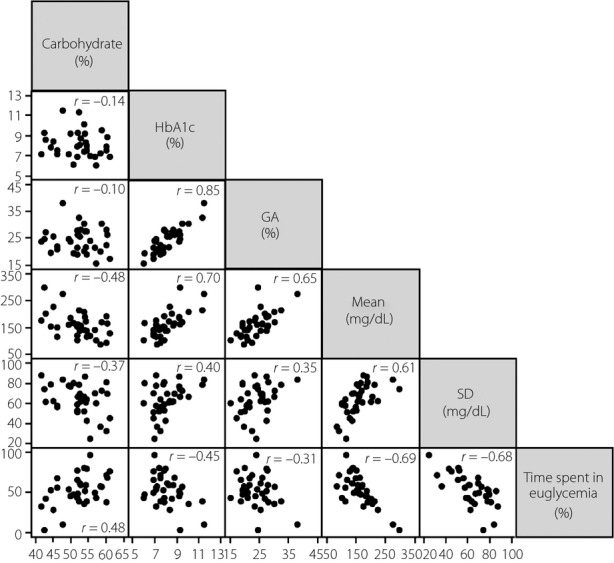
Relationship between carbohydrate intake and glycemic indices including glycated hemoglobin (HbA1c), glycated albumin (GA), mean calculated from continuous blood glucose monitoring data, standard deviation calculated from continuous blood glucose monitoring data and time spent in euglycemia calculated from continuous blood glucose monitoring data. SD, standard deviation.
Discussion
The present study shows a clear association of carbohydrate intake and favorable glycemic control during a consecutive 2-day period in a daily life setting of type 1 diabetes patients. Notably, carbohydrate intake showed no association with HbA1c or GA in the present study, showing that the association of carbohydrate intake with euglycemia does not represent a long-term interaction, but rather a short-term interaction, on a daily basis.
The existing evidence of the association of carbohydrate intake and glycemic control was estimated by food frequency questionnaires, which assesses long-term nutritional exposure, and HbA1c12,26, and there has been no association shown between carbohydrate intake estimated by dietary records, which assesses short-term nutritional exposure, and HbA1c27,28. In the present study, carbohydrate intake was calculated by dietary record during continuous monitoring of glucose levels. Mean glucose level during monitoring is generally the best index for correlating CGM with HbA1c, and showed an association with carbohydrate intake, whereas HbA1c did not. One reason could be that carbohydrate intake estimated by dietary record is not a good measure of chronic exposure to carbohydrate.
There was no trend found between carbohydrate intake and time spent in hypoglycemia, but a significant inverse association between carbohydrate intake and time spent in hyperglycemia was observed. Carbohydrate intake in the participants of the present study had a strong inverse correlation with fat intake, and the contributions of carbohydrate intake and fat intake to time in hyperglycemia and time in euglycemia were in the opposite direction. The contributions of carbohydrate intake were larger than those of fat intake. In contrast, protein intake showed no association with time in any range. The present results show that there is a relationship between increased carbohydrate intake or reduced fat intake and better short-term glucose control, which is less time in hyperglycemia and more time in euglycemia.
Insulin dose is also an important factor influencing glycemic control in type 1 diabetes, and is reported to correlate with carbohydrate intake28–30. We included insulin dose in the multiple regression models and observed an independent negative association between basal insulin dose and time spent in euglycemia, and an independent positive association between bolus insulin dose and time spent in hypoglycemia, but no association between insulin dose and time spent in hyperglycemia. This might suggest that higher insulin dose per bodyweight is a risk for hypoglycemia. CSII use was another factor associated with time spent in hypoglycemia, and also a factor reducing the time spent in hyperglycemia. CSII, therefore, could contribute to reduce hyperglycemia, but might tend to increase hypoglycemia, possibly more so under tight glycemic control.
Diabetes duration independently predicted euglycemia; patients with longer duration spent a longer time in euglycemia. Given that the CGM data in the present study were not shown to the patients in real time, they were assumed to manage their glycemic level based on self-monitoring of blood glucose and their experience. Patients might become skilled in managing their glycemic fluctuation through their long experience. Carbohydrate counting was used by seven out of the 33 patients on the days that glucose was monitored. Carbohydrate counting seemed to show a weak contribution to increased time spent in euglycemia, but the contribution did not reach statistical significance. Among the other plausible predictors that we included in the multiple regression models, fiber intake showed a tendency to reduce hypoglycemia, but the contribution was not significant when it was included in the model together with CSII use and insulin dose. The C-peptide level was undetectable by highly sensitive measurement in most patients in the present study, even after glucagon stimulation, and a dummy variable for C-peptide did not show a significant effect on time in target range or other indicators of glycemic variability. Hypoglycemia-associated autonomic failure can also increase hypoglycemia10. However, in our data, CVR-R was not an independent predictor of time spent in hypoglycemia. One reason for this could be that the CVR-R used in this study was not under deep breathing, but only the resting condition. Another limitation of the present study was the relatively small sample size.
A strength of our study was that our carbohydrate intake measurement did not reflect dietary history, but only the amount of carbohydrate actually taken during the period that the glucose level was continuously monitored. Time spent in a target glycemic range that includes both mean glucose level and glucose excursions on a daily basis is a suitable index for facilitation of glycemic control. We carried out additional analyses using other indexes of glycemic variability, including mean amplitude of glycemic excursions, coefficient of variation and mean absolute glucose change, but there were no significant findings.
Our data showed that carbohydrate intake ranged from 40% to 60% of total energy intake, whereas the guideline recommends that carbohydrate account for 50–60% of intake31. Patients who consumed carbohydrate accounting for <50% of total energy spent a shorter time in euglycemia and a longer time in hyperglycemia. This could partly be due to the fact that the insulin regimen was based mainly on carbohydrate intake, which is not appropriate for a relatively high-fat diet. Although fat content has been considered more important in controlling bodyweight than in controlling glycemic level11, fat intake delays the postprandial rise in blood glucose and makes peak glucose occur later32. It might therefore contribute to the mismatch of the insulin regimen and the glycemic level, and be a cause of glycemic variability and unpredictability. Further research is required to confirm these findings in experimental settings focusing on postprandial glucose or in other groups of patients whose intake is of a different composition of the three major nutrients from that of the present study.
In conclusion, sufficient carbohydrate intake is clearly associated with favorable glycemic control in patients with type 1 diabetes using MDI or CSII.
Acknowledgments
The present study was supported by Integration research for agriculture and interdisciplinary fields, Japan.
Disclosure
NI served as a medical adviser for Medtronic. The other authors declare no conflict of interest.
References
- Sassa M, Yamada Y, Hosokawa M, et al. Glycemic instability in type 1 diabetic patients: possible role of ketosis or ketoacidosis at onset of diabetes. Diabetes Res Clin Pract. 2008;81:190–195. doi: 10.1016/j.diabres.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Sartore G, Chilelli NC, Burlina S, et al. The importance of HbA1c and glucose variability in patients with type 1 and type 2 diabetes: outcome of continuous glucose monitoring (CGM) Acta Diabetol. 2012;49:S153–S160. doi: 10.1007/s00592-012-0391-4. [DOI] [PubMed] [Google Scholar]
- Kuenen JC, Borg R, Kuik DJ, et al. Does glucose variability influence the relationship between mean plasma glucose and HbA1c levels in type 1 and type 2 diabetic patients? Diabetes Care. 2011;34:1843–1847. doi: 10.2337/dc10-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelaar SE, Holleman F, Hoekstra JB, et al. Glucose variability; does it matter? Endocr Rev. 2010;31:171–182. doi: 10.1210/er.2009-0021. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Kidd J, Burmiston S, et al. Determinants of glycaemic control in type 1 diabetes during intensified therapy with multiple daily insulin injections or continuous subcutaneous insulin infusion: importance of blood glucose variability. Diabetes Metab Res Rev. 2006;22:232–237. doi: 10.1002/dmrr.614. [DOI] [PubMed] [Google Scholar]
- Ayano-Takahara S, Ikeda K, Fujimoto S, et al. Glycemic variability is associated with quality of life and treatment satisfaction in patients with type 1 diabetes. Diabetes Care. 2015;38:e1–e2. doi: 10.2337/dc14-1801. [DOI] [PubMed] [Google Scholar]
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SM, Kim TH, Bae JC, et al. Clinical factors associated with absolute and relative measures of glycemic variability determined by continuous glucose monitoring: an analysis of 480 subjects. Diabetes Res Clin Pract. 2014;104:266–272. doi: 10.1016/j.diabres.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Maahs DM, Mayer-Davis E, Bishop FK, et al. Outpatient assessment of determinants of glucose excursions in adolescents with type 1 diabetes: proof of concept. Diabetes Technol Ther. 2012;14:658–664. doi: 10.1089/dia.2012.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37:S120–S143. doi: 10.2337/dc14-S120. [DOI] [PubMed] [Google Scholar]
- Delahanty LM, Nathan DM, Lachin JM, et al. Association of diet with glycated hemoglobin during intensive treatment of type 1 diabetes in the Diabetes Control and Complications Trial. Am J Clin Nutr. 2009;89:518–524. doi: 10.3945/ajcn.2008.26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther. 2009;11:S55–S67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- Garg S, Jovanovic L. Relationship of fasting and hourly blood glucose levels to HbA1c values: safety, accuracy, and improvements in glucose profiles obtained using a 7-day continuous glucose sensor. Diabetes Care. 2006;29:2644–2649. doi: 10.2337/dc06-1361. [DOI] [PubMed] [Google Scholar]
- Williamson DA, Allen HR, Martin PD, et al. Comparison of digital photography to weighed and visual estimation of portion sizes. J Am Diet Assoc. 2003;103:1139–1145. doi: 10.1016/s0002-8223(03)00974-x. [DOI] [PubMed] [Google Scholar]
- Resources Council, Science and Technology Agency. Standard Tables of Food Composition in Japan. Tokyo: National Printing Bureau; 2005. . 5th revised and enlarged edition. [Google Scholar]
- Takhashi K, Yoshimura Y, Kaimoto T, et al. Validation of a food frequency questionnaire based on food groups for estimation individual nutrient intake. Jpn J Nutr Diet. 2001;59:221–232. [Google Scholar]
- Kumahara H, Schutz Y, Ayabe M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235–243. doi: 10.1079/BJN20031033. [DOI] [PubMed] [Google Scholar]
- Health Promotion and Nutrition Division, Health Service Bureau, Ministry of Health and Welfare, Japan. Recommended Dietary Allowance for Japanese. Tokyo: Dai-ichi Shuppan; 1994. , 5th revision. [Google Scholar]
- Hayano J, Sakakibara Y, Yamada A, et al. Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. Am J Cardiol. 1991;67:199–204. doi: 10.1016/0002-9149(91)90445-q. [DOI] [PubMed] [Google Scholar]
- Yoshinari M, Wakisaka M, Nakamura U, et al. Orthostatic hypertension in patients with type 2 diabetes. Diabetes Care. 2001;24:1783–1786. doi: 10.2337/diacare.24.10.1783. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ, Castillo MJ, Lefèbvre PJ. Assessment of residual insulin secretion in diabetic patients using the intravenous glucagon stimulatory test: methodological aspects and clinical applications. Diabetes Metab. 1996;22:397–406. [PubMed] [Google Scholar]
- Clarke WL, Cox DJ, Gonder-Frederick LA, et al. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517–522. doi: 10.2337/diacare.18.4.517. [DOI] [PubMed] [Google Scholar]
- Kawasaki E, Maruyama T, Imagawa A, et al. Diagnostic criteria for acute-onset type 1 diabetes mellitus (2012): report of the committee of Japan Diabetes Society on the research of fulminant and acute-onset type 1 diabetes mellitus. J Diabetes Investig. 2014;5:115–118. doi: 10.1111/jdi.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki C, Uchigata Y, Takaike H, et al. Intake of three major nutrients, body weight, and glycemic control in type 1 diabetes. J Japan Diab Soc. 2012;55:6–11. (Japanese) [Google Scholar]
- Brazeau AS, Mircescu H, Desjardins K, et al. Carbohydrate counting accuracy and blood glucose variability in adults with type 1 diabetes. Diabetes Res Clin Pract. 2013;99:19–23. doi: 10.1016/j.diabres.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Hamad S, Chiasson JL, et al. Day-to-day consistency in amount and source of carbohydrate intake associated with improved blood glucose control in type 1 diabetes. J Am Coll Nutr. 1999;18:242–247. doi: 10.1080/07315724.1999.10718858. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kawamura T, Kashihara Y, et al. Factors associated with basal insulin dose in Japanese children and young adult type 1 diabetics. J Diabetes Investig. 2012;3:276–282. doi: 10.1111/j.2040-1124.2011.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabasa-Lhoret R, Garon J, Langelier H, et al. Effects of meal carbohydrate content on insulin requirements in type 1 diabetic patients treated intensively with the basal-bolus (ultralente-regular) insulin regimen. Diabetes Care. 1999;22:667–673. doi: 10.2337/diacare.22.5.667. [DOI] [PubMed] [Google Scholar]
- The Japan Diabetes Society. Evidence-based practice guideline for the treatment of diabetes in Japan 2013. Tokyo, Japan, Nankodo, 2013 (Japanese) [article online (English)] Available from http://www.jds.or.jp/modules/en/index.php?content_id=44.
- Gentilcore D, Chaikomin R, Jones KL, et al. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab. 2006;91:2062–2067. doi: 10.1210/jc.2005-2644. [DOI] [PubMed] [Google Scholar]


