Currently, two types of cannula are available for Medtronic® insulin pump therapy: the vertical Quick-set® cannula (Medtronic plc, Dublin, Ireland) and the 30-degree angle Silhouette® cannula (Medtronic plc). However, it has not been established which cannula should be used in specific patients. Here, we present two Japanese patients with type 1 diabetes who experienced unexpected episodes of hyperglycemia caused by bending of the vertical cannula during insulin pump therapy.
Case 1: An 84-year-old Japanese woman (body mass index 18.7 kg/m2) who had fulminant type 1 diabetes caused by drug-induced hypersensitivity syndrome was admitted to Kanazawa University Hospital, Kanazawa, Ishikawa, Japan, because of repeated hyper- and hypoglycemic episodes. Her serum and urinary C-peptide were undetectable. We started a continuous subcutaneous insulin infusion (CSII) for better glycemic control. Initially, she used a 9-mm long Quick-set® cannula with the infusion set. However, she experienced several episodes of unexpected hyperglycemia caused by bending of the cannula tip and agglutination, even after switching to a 6-mm long Quick-set® cannula (Figure1). Therefore, she switched to a 13-mm long Silhouette® cannula inserted at a 30° angle. Subsequently, she did not experience the hyperglycemic episodes caused by obstruction of the cannula.
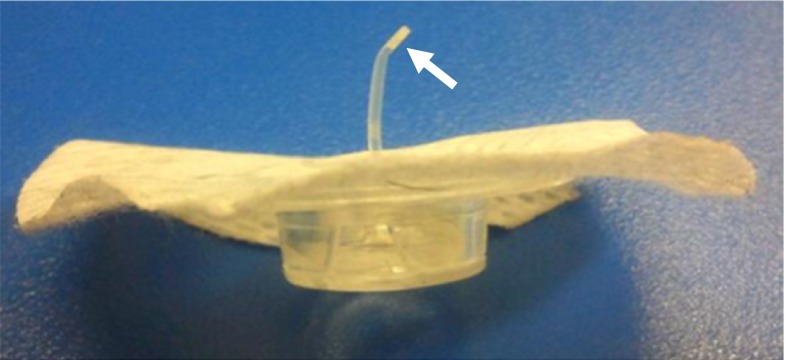
Figure 1.
A bent cannula tip and agglutination (arrow) in a 6-mm long Quick-set® cannula.
Case 2: A 57-year-old Japanese man (body mass index 21.3 kg/m2) who had had type 1 diabetes for 7 years was admitted to our hospital for control of brittle diabetes. His blood glucose had increased recently, and he had experienced some hypoglycemic episodes. He started CSII, using a 9-mm long Quick-set® cannula. He experienced some unexpected hyperglycemic episodes, without the insulin pump triggering an alarm, caused by cannula bending, which disappeared after switching to the Silhouette® cannula.
The two patients experienced a total of eight episodes of hyperglycemia. Of these, we recognized a bent cannula in six episodes (one with intracannula agglutination), and a normal cannula appearance in two episodes. Insertion failure or extended indwelling of the cannula alone do not explain the cannula problem, because four of the eight hyperglycemic episodes occurred 24–72 h after cannula insertion, unlike previous observations that most catheter problems start on day 31. We speculate that the vertical cannula tip reached the abdominal wall and bent during use because both of our patients were thin (skinfold thickness 8 mm in case 1 and 10 mm in case 2). Although the 6-mm long cannula was shorter than the skinfold thickness in case 1, there is a difference between the real skinfold thickness on insertion and the thickness on computed tomography. The cannula bends only on reaching abdominal muscle. Elderly people find it difficult to insert vertical cannulas, and they might not have inserted the cannulas adequately. In addition, a warning should be issued regarding the absence of a pump alarm, which occurred in one of the eight episodes.
We conclude that a bent vertical cannula tip should be considered to be a cause of unexpected hyperglycemic episodes during CSII therapy, especially in relatively slender people with diabetes. A prospective randomized controlled study comparing the outcomes of 9 mm versus 6 mm and vertical versus horizontal cannulas in type 1 diabetic patients with different body mass indexes, body fat percentages and skinfold thicknesses is necessary to prove our hypothesis.
Furthermore, the alarm system of the CSII device should be improved, so that it detects early cannula obstruction to prevent unexpected hyperglycemic episodes.
Disclosure
The authors declare no conflict of interest.
Reference
- Schmid V, Hohberg C, Borchert M, et al. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy—trouble starts on day 3. J Diabetes Sci Technol. 2010;4:976–982. doi: 10.1177/193229681000400429. [DOI] [PMC free article] [PubMed] [Google Scholar]