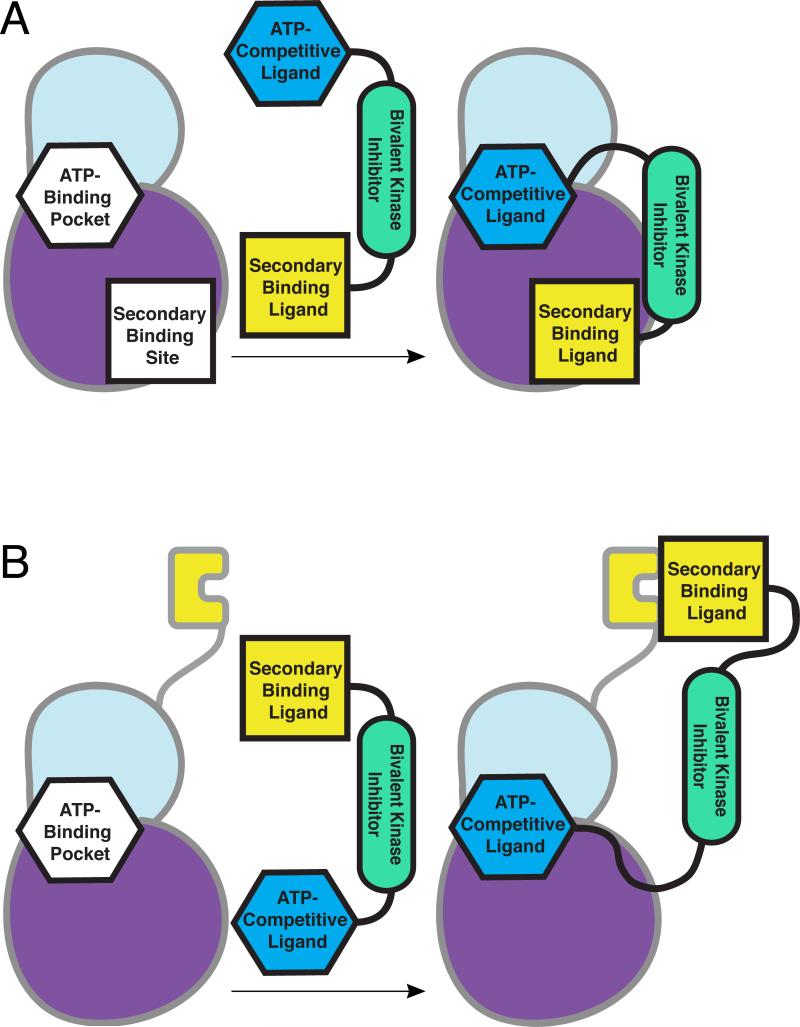
Figure 1.
Bivalent inhibition of a protein kinase. (a) A single inhibitor is generated by fusing an ATP-competitive ligand to a secondary binding ligand. The ATP-competitive ligand interacts with the kinase active site, directing the bivalent inhibitor to the kinase. The secondary binding ligand introduces selectivity for a particular kinase. In the case of bisubstrate inhibitors, this ligand targets the protein substrate site of the protein kinase. (b) Regulatory domains of protein kinases present an avenue for bivalent inhibition where secondary-binding ligands can target domains distal to the kinase domain.