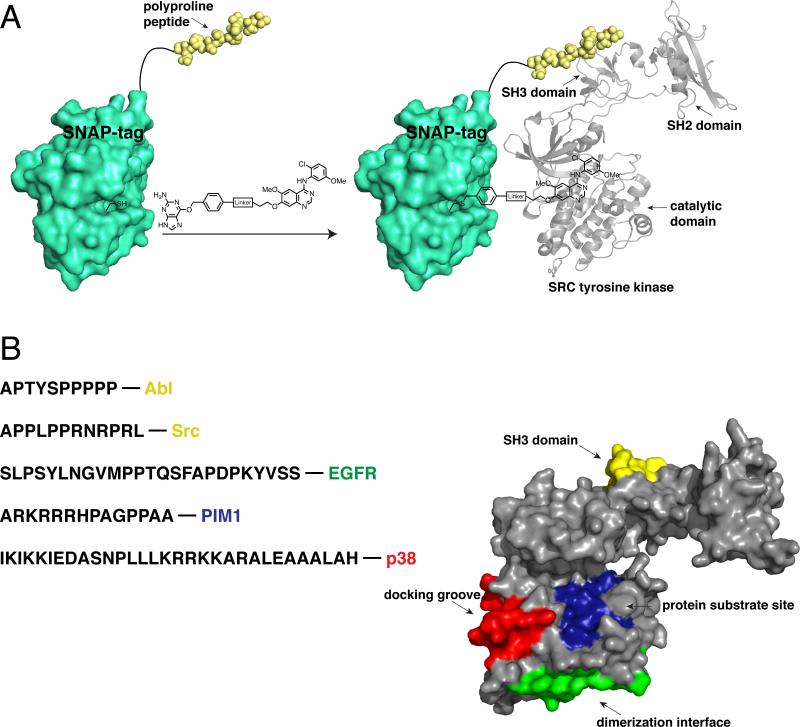
Figure 3.
A SNAP-tag-based bivalent inhibitor approach. (a) Schematic depiction of a representative SNAP-tag-based bivalent inhibitor. SNAP-tag self-labels an active site cysteine with a quinazoline-based kinase inhibitor conjugated to a benzylguanine (BG) group. The genetically encoded SNAP-tag fusion contains a polyproline peptide ligand, which recognizes the SH3 domain of Src. The two ligands of the bivalent inhibitor interact with distinct Src tyrosine kinase binding sites. [PDB codes 3L00 and1Y57] (b) The sequences of peptide ligands that have been successfully used in SNAP-tag-based bivalent/bisubstrate inhibitors, and the kinases they target, are shown. The binding sites that have been targeted with SNAP-tag-based bivalent inhibitors are shown. These sites are superimposed on a crystal structure of Src tyrosine kinase. [PDB code 1Y57]