Fig 9. Model describing oxidative stress response in Klotho responsive cells and tissues.
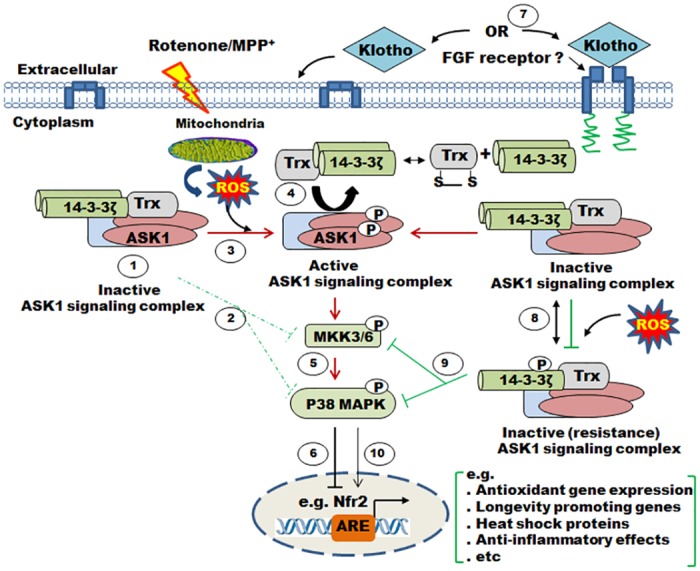
(1–2) In normal unstressed cells and tissues, 14-3-3ζ and thioredoxin cooperate with other cytosolic proteins to inhibit the ASK1 signaling complex with concomitant downstream reduction in MAPKs phosphorylation level. (3–6) In an oxidizing environment generated by reactive oxygen species (ROS) via mitochondrial electron transport chain dysfunction induced by rotenone, ASK1 dissociates from thioredoxin/14-3-3ζ, becomes phosphorylated (activating ASK1 signaling compex) and, in turn, phosphorylates downstream MAPKs, including p38. These cascades of events inhibit nuclear translocation of transcription factors such as Nrf2, leading to suppression of antioxidant and longevity-promoting genes. Puzzling is our observation that 14-3-3ζ and thioredoxin remained partially bound. (7–10) By contrast, 14-3-3ζ interaction with the ASK1 signaling complex is consolidated by Klotho-dependent phosphorylation of the 14-3-3ζ at Ser-58, promoting monomer formation; 14-3-3ζ monomerization may increase its interaction with the inhibitory ASK1 by forming a stable complex. This activity is expected to facilitate Nrf2 nuclear localization promoting expression of antioxidant and longevity-promoting genes.
