Abstract
Mild traumatic brain injury (mTBI) is common in the United States, accounting for as many as 75–80% of all TBIs. It is recognized as a significant public health concern, but there are ongoing controversies regarding the etiology of persistent symptoms post-mTBI. This constellation of nonspecific symptoms is referred to as postconcussive syndrome (PCS). The present study combined results from magnetoencephalography (MEG) and cognitive assessment to examine group differences and relationships between brain activity and cognitive performance in 31 military and civilian individuals with a history of mTBI+PCS and 33 matched healthy control subjects. An operator-free analysis was used for MEG data to increase reliability of the technique. Subjects completed a comprehensive neuropsychological assessment, and measures of abnormal slow-wave activity from MEG were collected. Results demonstrated significant group differences on measures of executive functioning and processing speed. In addition, significant correlations between slow-wave activity on MEG and patterns of cognitive functioning were found in cortical areas, consistent with cognitive impairments on exams. Results provide more objective evidence that there may be subtle changes to the neurobiological integrity of the brain that can be detected by MEG. Further, these findings suggest that these abnormalities are associated with cognitive outcomes and may account, at least in part, for long-term PCS in those who have sustained an mTBI.
Key words: : executive functioning, magnetoencephalography (MEG), mild traumatic brain injury (mTBI), neuropsychological testing, postconcussive syndrome (PCS)
Introduction
Over 1.7 million traumatic brain injuries (TBIs) are reported in the United States every year, making TBI a significant public health concern.1,2 TBI is graded by severity based on initial injury variables, such as loss of consciousness. Severity of brain injuries range from mild to severe, although 75–80% of TBIs treated in hospitals are classified as mild. Given that many individuals with mild injuries do not seek immediate medical care, incidence of mild TBI (mTBI) may be much higher.3 The cause of mTBI among civilians is varied and includes motor vehicle accidents (MVAs), sport-related injuries, falls, and assaults. Blast exposure represents an additional cause of mTBI among U.S. troops in combat as part of ongoing military operations in the Middle East. Large-scale postdeployment surveys have consistently reported that 15–20% of service members may have suffered an mTBI during deployment, the majority related to blast exposure.4,5
Although studies have indicated that acute symptoms of mTBI resolve rapidly in most patients, some individuals with mTBI continue to experience symptoms in the months and even years after the initial injury.6–13 Debate about the etiology of these persistent symptoms, referred to as postconcussive syndrome (PCS), continues. Various studies that included neuropsychological testing have reported reduced cognitive efficiency, especially on tests measuring executive functioning, processing speed, attention, and memory in patients with mTBI and persistent PCS.14–16 However, others have suggested that the observed impairments on neuropsychological tests are of insufficient severity to be clinically significant.17,18 In addition, controversy exists as to whether the persistent symptoms and cognitive impairments reported in some studies are evidence of residual neuropathological effects of the injury or are owing to motivational issues (i.e., secondary gain) or psychological factors.19 Because conventional neuroimaging (computed tomography or magnetic resonance imaging [MRI]) studies are normal in most individuals with an mTBI, it is likely that this debate will continue until more sensitive, objective measures of brain function and structure are developed.20–25 Variability in the case definition of mTBI, differences in outcome measures, and a lack of a validated diagnostic gold standard further complicate interpretation of published studies and contribute to the continuing debate.26,27
One approach to resolving this controversy is to combine findings from multiple tests in an attempt to improve diagnostic clarity and investigate relationships between imaging- and performance-based functional measures.28 By including new neuroimaging modalities and results from neuropsychological testing, it may be possible to provide objective evidence of long-term neuropathological changes associated with mTBI. If a combination of tests and measures can accurately identify individuals at risk for chronic symptomatology early, timely and more accurate targeting of interventions may occur to improve clinical outcomes among this subset of mTBI patients. In addition, the use of highly sensitive measures of brain function will help further elucidate potential neuropathological causes of PCS. Ultimately, this approach can reduce the long-term functional and economic effects of persistent PCS post-TBI by improving the reliability and accuracy of diagnosis.
In 2007, Lewine and colleagues. analyzed results from clinical neuroimaging studies, magnetoencephalography (MEG), single-photon emission computed tomography (SPECT), and neuropsychological testing in a study of a clinical sample of patients with mTBI and PCS. Based on the results, they argued that subtle changes in the neurobiological integrity of the brain may account for PCS post-mTBI.28 Results from MEG were particularly intriguing because of potential effects of motivational and compensation-related factors in this population. Whereas it is common to utilize activation tasks to study cognitive domains, data from “resting-state” recordings from MEG and SPECT require no effort from the participant and are thus less susceptible to motivational influences. In addition, resting-state imaging can be used as a passive measure of global integrity of the brain without a priori knowledge regarding which regions of activation are related to specific tasks.28 In Lewine and colleagues' study, resting-state measures of brain activity derived from MEG revealed abnormalities of the electrophysiological integrity of the brain in a large percentage of patients with persistent cognitive symptoms post- mTBI. In fact, 86% of this sample demonstrated abnormalities on resting-state MEG, compared to 40% with abnormalities on SPECT, and only 18% showing changes on clinical MRI. Lewine and colleagues also reported that MEG slow-wave abnormalities correlated significantly with impairments on neuropsychological tests. They found that temporal lobe slowing correlated with memory impairments and decreased processing speed, parietal slowing with attentional impairments, and frontal slowing with impairments of executive functioning. However, the investigators' ability to attribute these correlations to the injury was limited by the lack of an uninjured control group. In addition, the approach from Lewine and colleagues is based on a dipole modeling (>0.80 goodness of fit). Their approach did not fit MEG signal generated from non- or multi-dipolar sources. In contrast, the VESTAL approach, developed and demonstrated by Huang and colleagues, provides MEG source images for dipolar, multi-dipolar, as well as nondipolar sources.29 The VESTAL approach can also resolve both uncorrelated, as well as 100% temporally correlated, sources. Another difference between the approach from Lewine and colleagues and the VESTAL approach is that VESTAL examines the total root mean square (RMS) across an entire recording session, whereas the approach from Lewine and colleagues focuses on a subset of the data session with large sensor waveform magnitudes with a predetermined threshold (i.e., 200 fT for sensor waveform amplitude).
In 2012, Huang and colleagues utilized the novel MEG low-frequency source imaging VESTAL approach in participants with mild-to-moderate TBI.30 Using this approach, resting delta slow-wave activity was examined in 45 participants with mTBI and persistent PCS, 10 participants with moderate TBI and persistent PCS, and 44 healthy controls (HCs). Abnormal delta slowing was detected in 87% of participants with mTBI and 100% with moderate TBI. This abnormal pattern of slowing was not found to be present in any of the HC participants. Inclusion of neurologically intact controls and use of an examiner-free method of data processing provides objective evidence that this pattern of slow-wave activity is not a normal variant. The number of cortical areas exhibiting abnormal slow-wave activity was additionally correlated with the number of reported symptoms that participants endorsed. The results from the Lewine and colleagues and Huang and colleagues studies provide evidence that subtle electrophysiological abnormalities may contribute to continued symptoms post-mTBI.
The current study extends the findings from the Lewine and colleagues and Huang and colleagues studies. In addition, we aim to further characterize the relationships between performance on neuropsychological tests and slow-wave activity on MEG in participants with mTBI and persistent PCS. Participants with mTBI occurring at least 3 months before enrollment and reporting at least three persistent PCS were included in the present analysis (mTBI+PCS). HC participants with no history of TBI or other neurological disorders affecting the brain were also recruited for comparison. All participants completed a battery of standardized neuropsychological measures and underwent a number of neuroimaging evaluations, including MEG, as part of their participation in the study. Correlations between outcomes of slow-wave detection using MEG and performance on neuropsychological measures were calculated to examine regional changes in brain functioning. Results from the neuropsychological evaluation were used to compare cognitive performance of the mTBI+PCS group to that of the HC group. This study was designed to examine three primary hypotheses: 1) Using a battery of standard neuropsychological tests, performance of the mTBI+PCS group will be significantly worse than the performance of a well-matched HC group; 2) slow-wave activity, as measured by MEG, will be significantly correlated with worse performance on neuropsychological measures; and 3) abnormal slow-wave activity will be significantly correlated to cortical regions known to mediate performance on the specific cognitive measures.
Methods
Recruitment
Sixty-four participants were recruited as part of a larger cohort study on TBI using standardized and approved subject recruitment procedures.29,30 A convenience sample of Veterans with mTBI resulting from blast exposure, MVAs, or sports injuries were recruited through primary care physicians and the Traumatic Brain Injury Clinic at the Veterans Administration San Diego Health Care System La Jolla. Active duty military participants with mTBI resulting from blast or accidents were recruited from the Defense and Veterans Brain Injury Clinic at the Naval Medical Center San Diego. A convenience sample of civilian participants with mTBI were recruited through the University of California San Diego Medical Center Trauma Clinic and through San Diego Sports Medicine and Family Health Center. HC participants were recruited from the local community by ads and flyers. The study received institutional review board approval, and all participants provided informed consent according to Veterans Affairs, University of California San Diego, and Naval Medical Research Center requirements. The mTBI+PCS group was recruited first and the HC group was matched to the clinical group on demographic characteristics of age, education, and gender. Table 1 displays participants' demographic characteristics. The final sample included 31 participants with a history of mTBI, with a mean age of 26.6 years (standard deviation [SD]=6.1) and a mean education level of 12.8 years (SD=1.2). HCs constituted 33 participants in the final sample with a mean age of 26.3 years (SD=8.3) and a mean education level of 13.3 years (SD=1.5). The mTBI group included 90.3% male participants whereas the HC group included 93.9% male participants, with Caucasian participants being the majority of both groups. Of the mTBI group, 64% sustained injuries related to blast injury during combat, 16% were injured in MVAs, and 19% sustained sports-related injuries. The groups did not differ significantly on age and years of education; however, because scores on the Weschler Test of Adult Reading (WTAR; p values) and Personality Assessment Inventory Anxiety Related Disorders Traumatic Stress Subscale (PAI ARD-T; p values) differed significantly, these variables were used as covariates for further analyses.31,32
Table 1.
Participant Characteristics: mTBI+PCS and HCs
| Groups | |||
|---|---|---|---|
| mTBI+PCS M (SD) | HC M (SD) | p values | |
| Group size | 31 | 33 | |
| Gender (% male) | 90.3 | 93.9 | |
| Handedness (% right) | 93.5 | 93.9 | |
| Age at exam | 26.6 (6.1) | 26.3 (8.3) | 0.894 |
| Education | 12.8 (1.2) | 13.3 (1.5) | 0.199 |
| WTAR standard score | 96.13 (16.4) | 107.52 (12.59) | 0.003 |
| PAI ARD-T t score | 59.87 (13.05) | 49.48 (10.26) | 0.001 |
| Days postinjury | 97.4 (333.7) | N/A | |
Demographic means and standard deviations are shown. p<0.05 is held as significant.
mTBI+PCS, mild traumatic brain injury and postconcussive syndrome; WTAR, Weschler Test of Adult Reading; PAI ARD-T, Personality Assessment Inventory Anxiety Related Disorders Traumatic Stress Subscale; HC, healthy controls; M, mean; SD, standard deviation; N/A, not applicable.
mTBI was defined by standard diagnostic criteria to include a loss or altered state of consciousness for less than 15 min postinjury or a period of post-traumatic amnesia of less than 24 h or a Glasgow Coma Scale rating between 13 and 15.33,34 As part of the exclusion criteria, all participants underwent a structural MRI (1.5T GE scanner; GE Healthcare, Woburn, MA) with a standardized TBI sequence, including a sagittal magnetization-prepared rapid acquisition gradient echo, axial gradient-echo T2*, axial fluid-attenuated inversion recovery, axial T2, and axial diffusion-weighted imaging (DWI). In order to address potential confounds related to evidence of visible structural changes in the brain (e.g., related to previous injuries), MRI results were reviewed by a board-certified neuroradiologist. Results of the MRI had to be negative for the participant to remain in the mTBI+PCS or HC groups. In addition, available clinical MRI studies for the mTBI+PCS group conducted after the documented injury were examined and also had to be negative for blood product for the participant to qualify for inclusion in the study. Additional exclusionary criteria for all participants were a past history of neurological or psychiatric disorders. Participants were also excluded if they were on medications, such as neuroleptic sedatives, antidepressants, and hypnotics, that may globally increase delta slow-wave activity.35
All participants underwent a semistructured clinical interview regarding health and neurological history, a battery of neuropsychological tests, and neuroimaging evaluations, including MRI and MEG, at the University of California San Diego Radiology Imaging Lab. To address possible effects of effort and motivational factors on neuropsychological performance, the Test of Memory Malingering (TOMM) was administered to all subjects.36–38 In addition, patterns of performance and embedded indicators of effort were examined. One participant was excluded from analysis owing to questionable performance on the TOMM.
Neuropsychological tests
All neuropsychological tests were administered by trained research associates. The results were scored, double scored, and reviewed by a licensed neuropsychologist to maintain reliability. All evaluations were administered in a single session in a quiet room, within 1 week of the MEG and MRI scans. Tests were selected to assess a number of cognitive domains.39 The WTAR scaled score was used as an indicator of preinjury intellectual functioning and is included in the demographic comparison.31 The Personality Assessment Inventory (PAI) was administered as a measure of psychiatric functioning.32 As there are potential effects of co-occurring post traumatic stress disorder on cognitive tests, the PAI ARD-T t-score was included as a potential covariate.32 The California Verbal Learning Test (CVLT-II) and the Brief Visuospatial Memory Test (BVMT-R) were included as measures of verbal and visual learning and memory.40,41 Attention, working memory, and concentration were measured using the Letter/Number Sequencing and Digit Span subtests from the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) and the Connors Continuous Performance Test II (CPT-II).42,43 The Delis-Kaplan Executive Function System (D-KEFS) Sorting, Trail Making, Color-Word Interference, and Verbal Fluency subtests were used to evaluate executive functioning.44 Processing speed was assessed with the WAIS-III Processing Speed Index subtests of Symbol Search and Digit Symbol Coding.42 Finally, motor functioning was measured with the Grooved Pegboard.45,46 Age-corrected scaled scores or t scores from the test manuals were used as dependent measures in the analyses.
Statistical analysis of neuropsychological tests
Standard statistical analysis software (SPSS 12.0 for Windows; SPSS, Inc., Chicago, IL) was used for all neuropsychological data analysis. In order to determine whether the groups were matched, demographic, educational, and trauma-related anxiety characteristics of the mTBI+PCS and HC groups were compared using a univariate analysis of variance (ANOVA; see Table 1); characteristics that differed significantly (p<0.05) were used as covariates in further analyses. To protect against inflation of type 1 error rate, differences between the mTBI and HCs on the means of 19 neuropsychological dependent variables were evaluated using multivariate analysis of covariance (MANCOVA), followed by a series of univariate analyses of covariance (ANCOVAs) to establish which tests accounted for any significant effect found with the MANCOVA.47 Whereas data were collected for 22 different neuropsychological dependent variables, owing to 5 participants not completing the BVMT-R and D-KEFS Sorting subtests, and the high collinearity between the CVLT long-delay free recall variable of the CVLT-II with the other two CVLT-II variables, four variables were removed from the MANCOVA analysis. Box's Test of Equality of Covariance Matrices was used to make sure that the covariance matrices of the dependent variables between the two groups were equal. Pearson's correlations between the dependent variables were performed to test the MANCOVA assumption that the dependent variables would be correlated with one another in the moderate range (0.20–0.60).48 The Partial Eta Squared, ηp2, effect size was calculated for the adjusted analyses. Family-wise error rate was addressed using false discovery rate (FDR) correction for multiple comparisons, with resulting p value significance level calculated at p≤0.004.49 A list of the 18 variables included in the MANCOVA analysis can be found in Table 2 because those variables were also included in the ANCOVA analysis. The CVLT long-delay forced choice variable is included in Table 2, but was not a part of the MANCOVA analysis owing to its high collinearity with the other CVLT variables.
Table 2.
Neuropsychological Test Scores in ANCOVA Analysis
| Uncorrected means | mTBI+PCS vs. HC | mTBI+PCS vs. HC | |||
|---|---|---|---|---|---|
| Neuropsychological measure | mTBI+PCS M (SD) | HC M (SD) | Uncorrected Cohen's d | ANCOVA p values | Significant effect sizes ηp2 |
| WASI Verbal IQ | 102.4 (13.8) | 111.8 (13.7) | −0.684 | 0.285 | NS |
| WASI Performance IQ | 110.4 (12.0) | 108.9 (14.0) | 0.115 | 0.245 | NS |
| CVLT-II 1-5 Free Recall t score | 52.61 (8.5) | 55.67 (8.3) | −0.364 | 0.494 | NS |
| CVLT-II Short Delay Free Recall z-score | 0.24 (0.9) | 0.65 (0.7) | −0.509 | 0.039 | 0.069 |
| CVLT-II Long Delay Free Recall z-score | 0.03 (0.9) | 0.45 (0.9) | −0.467 | 0.158 | NS |
| WAIS-III Letter/Number Sequencing Scaled | 9.9 (2.1) | 10.8 (1.9) | −0.449 | 0.893 | NS |
| WAIS-III Digit Span Scaled | 10.7 (2.7) | 10.9 (2.3) | −0.080 | 0.691 | NS |
| CPT Inattention Omissions t-score | 58.4 (34.0) | 57.0 (32.7) | 0.042 | 0.893 | NS |
| CPT Inattention Commissions t-score | 49.6 (6.6) | 46.6 (9.1) | 0.378 | 0.261 | NS |
| D-KEFS Trails Number Letter Sequencing Scaled | 8.9 (2.4) | 11.27 (2.3) | −1.008 | 0.003*** | 0.138 |
| D-KEFS Color-Word Interference Inhibition Scaled | 9.16 (2.1) | 11.64 (2.4) | −1.100 | 0.004*** | 0.132 |
| D-KEFS Color-Word Interference Inhibition/Switching Scaled | 8.9 (2.6) | 10.4 (2.5) | −0.588 | 0.072 | NS |
| D-KEFS Verbal Fluency Letter Fluency Scaled | 8.9 (2.3) | 11.9 (2.7) | −1.196 | 0.002*** | 0.152 |
| D-KEFS Verbal Fluency Category Fluency Scaled | 10.7 (3.2) | 12.8 (2.6) | −0.720 | 0.091 | NS |
| D-KEFS Verbal Fluency Category Switching Scaled | 9.7 (2.6) | 11.0 (2.5) | −0.510 | 0.477 | NS |
| WAIS-III Symbol Search Scaled | 11.6 (2.4) | 12.6 (3.2) | −0.354 | 0.314 | NS |
| WAIS-III Digit Symbol Coding Scaled | 9.2 (2.5) | 11.7 (2.6) | −0.980 | 0.004*** | 0.132 |
| Grooved Pegboard Dominant Hand Scaled | 9.7 (2.0) | 9.7 (2.5) | 0.000 | 0.474 | NS |
| Grooved Pegboard Non-Dominant Hand Scaled | 10.0 (2.3) | 9.8 (2.9) | 0.344 | 0.569 | NS |
Neuropsychological test scores comparing mTBI+PCS and HC groups with uncorrected means, standard deviations, and Cohen's d as well as corrected ANCOVA adjusted analysis (p values) and significant main effects (ηp2).
p values indicating significance in ANCOVA adjusted analysis with FDR corrected p≤0.004. NS=no significant main effects found. All standardized scores are corrected for age and education level.
ANCOVA, analysis of covariance; WASI, Wechsler Abbreviated Scale of Intelligence; CVLT-II, California Verbal Learning Test; WAIS-III, Wechsler Adult Intelligence Scale, Third Edition; CPT, Connors Continuous Performance Test; D-KEFS, Delis-Kaplan Executive Function System; mTBI+PCS, mild traumatic brain injury and postconcussive syndrome; HC, healthy controls; M, mean; SD, standard deviation.
Magnetoencephalography data acquisition, processing, and analysis
MEG data were acquired during three 4-min resting-state (spontaneous recording for detecting low-frequency MEG signals), eyes-closed sessions for all participants with an Elekta/Neuromag™ whole-head MEG system (VectorView; Elekta Neuromag, Helsinki, Finland) with 204 gradiometers and 102 magnetometers in a magnetically shielded room (IMEDCO-AG, Hägendorf, Switzerland).50 Participants were instructed to keep their eyes closed and empty their minds. Electrooculography electrodes were used to detect eye blinks and eye movements. Electrocardiography electrodes were used to detect cardiac motion artifacts. Data were recorded spontaneously at 1000 Hz, with no signal averaging. To increase the likelihood that subjects would be alert during the MEG recordings, participants were given a questionnaire asking how many hours they slept the previous night, how rested they felt, and if there was any reason that they were not attentive and performing to the best of their abilities (owing to headache, pain, and so on); participants were rescheduled as necessary. In addition, eyes-closed sessions alternated with eyes-open sessions, during which MEG technicians were able to monitor eye blinking and closing as an indicator of subjects' cognitive alertness. During the session, MEG technicians could also monitor the amount of alpha-band oscillation, which is consistently associated with tonic alertness. Finally, MEG technicians were able to view participants using a nonrecording video camera during sessions to monitor level of alertness as well as safety.
After data collection, data were processed by MaxFilter to remove environment noise.51–54 A Realistic Boundary Element Method head model was used for MEG forward calculation.55,56 The BEM mesh was constructed by tessellating the inner skull surface from the T1-weighted MRI into ∼6000 triangular elements with ∼5-mm size. A cubic source grid with 5-mm size was used for calculating the MEG gain (i.e., lead-field) matrix, which leads to a grid with ∼7000 nodes covering the cortical and subcortical gray matter of the whole brain. Registration of MRI and MEG was performed using data obtained from the Polhemus Isotrak system before MEG scanning (Polhemus, Colchester, VT). MEG data were then band-pass filtered for 1–6 Hz, mainly focusing on the delta band. Source reconstruction of each session for each subject was performed using the Frequency-Domain VESTAL approach.30,57,58 RMS amplitude per grid point was then computed for each subject and saved in a three-dimensional Nifty format file, which was then interpolated into 1×1×1 images in native MR coordinates. RMS reconstructions in native scanner space were then smoothed (5-mm gaussian kernel) and averaged across all sessions for each subject. The averaged and smoothed set of activities was subsequently transferred to MNI 152 space for each subject using FLIRT registration with the FSL software package.59,60 The resulting reconstructions in MNI space were then correlated (voxel-by-voxel, 1×1×1 size) across subjects with neuropsychological measures that displayed significant group differences. Uncorrected p values were then corrected using FDR correction for multiple comparisons.49 Masks generated from voxels surviving FDR multiple comparisons across voxels were applied to the R-value volumes for each neuropsychological measure, and surviving voxels of the FDR correction are displayed in the figures shown. An additional clustering analysis was performed to confirm that the voxels that survived FDR correction were members of larger clusters, rather than rogue events. In the additional cluster analysis that corrected multiple comparisons across voxels, the size of the cluster for the corrected p<0.05 was determined by the AlphaSim program in AFNI. Each reported correlation that had an FDR corrected also was confirmed to be in a cluster of size >150 voxels with a p<0.05.61
Data processing stream for statistical analysis
Results from the cognitive assessments of the mTBI+PCS and HC groups were compared to determine whether there were any differences between groups. Once the final univariate analysis was performed on specific neuropsychological dependent variables, those showing significant group differences were selected for inclusion in a MEG correlational analysis, in order to determine whether slow-wave RMS amplitude correlated with performance on neuropsychological tests, particularly in regions known to mediate performance on specific cognitive measures.
Results
Cognitive functioning
A major aim of this study was to determine whether participants with a history of mTBI and PCS lasting for at least 3 months postinjury would show worse performance than controls on a battery of cognitive tests. Before performing the MANCOVA, a Pearson's correlations matrix was generated and showed a meaningful pattern of correlations, with many of the variables moderately correlated with one another (r value between 0.20 and 0.60), suggesting the appropriateness of a MANCOVA.48
The Box's M value of 271.787 was associated with a nonsignificant p value of 0.195, also supporting appropriateness of MANCOVA. A MANCOVA with group as the independent variable and scores from the neuropsychological tests as the dependent variables yielded a statistically significant main effect for group (Wilks'lambda=0.515; F(18,43)+2.249; p<0.05; partial η2=0.485), with the mTBI+PCS participants performing worse than the demographically matched controls. The effect size of 0.485 suggests that 48.5% of the canonically derived dependent variable was owing to group membership.
Post-hoc comparisons (Table 2) revealed statistically significant group differences on the following measures from the D-KEFS with the mTBI+PCS group showing worse performance: the D-KEFS Trail-Making subtest Number Letter Sequencing scaled score (F(1,60)=9.631; p=0.003; ηp2=0.138), the D-KEFS Color-Word Interference subtest Inhibition scaled score (F(1,60)=9.125; p=0.004; ηp2=0.132) and the D-KEFS Verbal Fluency subtest Letter Fluency scaled score (F(1,60)=10.778; p=0.002; ηp2=0.152). There was also a nonsignificant trend toward significance on the D-KEFS Color-Word Interference subtest Inhibition Switch scaled score (F(1,60)=3.346; p=0.072) with effect (ηp2=0.053). In addition, performance of the groups differed significantly for a task measuring processing speed, the Digit Symbol Coding subtest (WAIS III; (F(1,60)=9.085; p=0.004; ηp2=0.132).
There was limited evidence of any group differences on measures of learning and recall, although differences in group performances on the CVLT-II Short Delay Free Recall trial were suggestive of a trend, with the mTBI+PCS group showing worse performance (F(1,60)=4.461; p=0.039) with a nonsignificant effect (ηp2=0.069). There were no further group differences found in the neuropsychological variables. Mean performance of both groups on all measures fell within the average to high average range (see Table 2).
Magnetoencephalography data correlational results
The neuropsychological variables showing significant group differences or trends toward significance were correlated with the results from the MEG slow-wave evaluation. These variables included: D-KEFS Trail Making Number Letter Sequencing scaled score; D-KEFS Color-Word Interference Inhibition scaled score and Inhibition Switch scaled score, D-KEFS Verbal Fluency Letter Fluency scaled score; WAIS-III Digit Symbol Coding scaled score; and, finally, the CVLT-II Short Delay Free Recall z-score. Rogue slow waves can be found to occur rarely in HC participants, though not at the frequency and amplitude reported in previous studies of mTBI patients. In order to address this potential issue, the MEG data from all healthy controls were also run through the MEG correlational analysis paradigm and no significant correlations were found.
Results of the correlational analysis are shown in Figures 1–3. Images are all in radiological convention and displayed as right on left. Three of the variables were found to have significant correlations between the slow-wave RMS amplitude and the mTBI population test scores. The structural locations were obtained using the Harvard-Oxford Cortical Structural Atlas from FSL 4.1.3.62–66
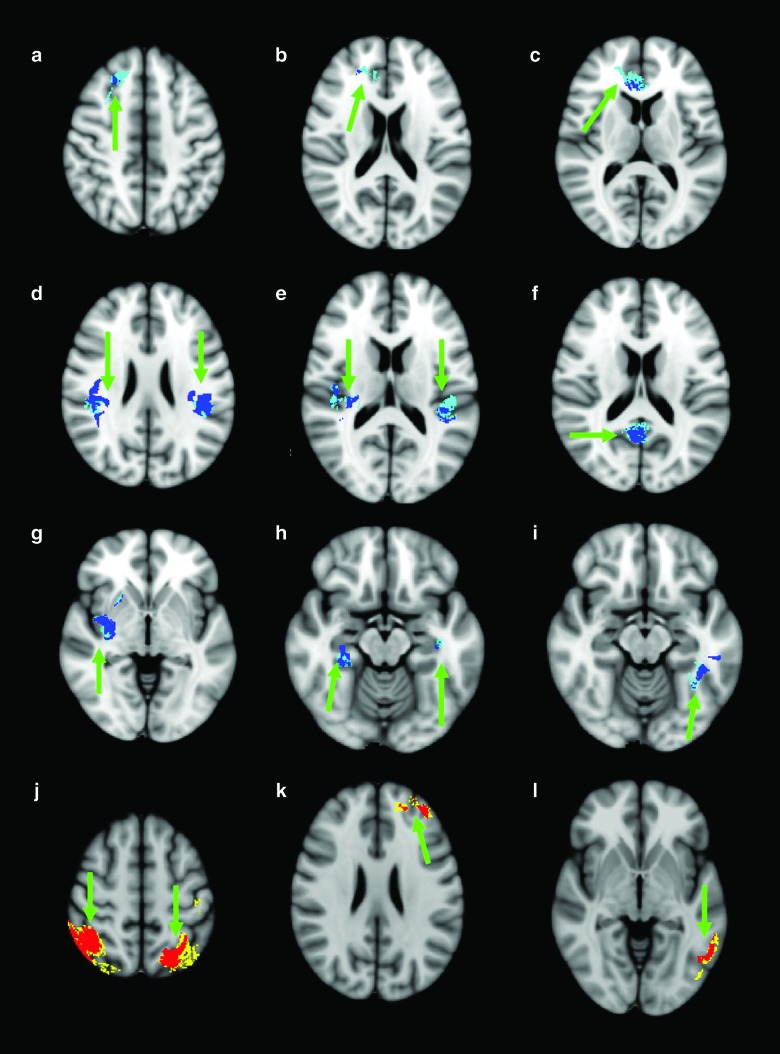
FIG. 1.
Images are displayed right on left, using the Harvard-Oxford Cortical Structural Atlas from FSL 4.1.3 for reference. Dark blue regions focused on in (a)–(i) demonstrate significant regions that have passed false discovery rate (FDR) correction (indicated by green arrow) associated with a negative correlation between slow-wave root mean square (RMS) amplitude and performance on Delis-Kaplan Executive Function System (D-KEFS) Color Word Interference Inhibition Scaled (i.e., poorer performance on task with higher slow-wave amplitudes). Teal regions surrounding the dark blue regions indicate where an additional cluster analysis was performed to confirm the regions that passed FDR correction. Red regions focused on in (j)–(l) demonstrate significant regions that have passed FDR correction (indicated by green arrow) associated with a positive correlation between slow-wave RMS amplitude and performance on D-KEFS Color-Word Interference Inhibition Scaled (i.e., better performance on tasks with a higher slow-wave amplitude). Yellow regions surrounding the red regions indicate where an additional cluster analysis was performed to confirm the regions that passed FDR correction. (a) Right superior/middle frontal gyrus (r=0.511). (b) Right frontal pole (r=0.462). (c) Right anterior cingulate gyrus (r=0.472). (d) Bilateral inferior parietal lobe (parietal operculum and supramarginal gyrus), left (r=0.570) and right (r=0.520). (e) Bilateral planum temporale and Heschl's gyrus, including H1 and H2, left (r=0.475) and right (r=0.567). (f) Right precuneous cortex and cingulate gyrus (r=0.547). (g) Right insular cortex (r=0.492). (h) Bilateral hippocampus and parahippocampal gyrus left (r=0.452) and right (r=0.489). (i) Left temporal fusiform/inferior temporal gyrus (r=0.507). (j) Bilateral superior parietal lobe left (r=0.452) and right (r=0.489). (k) Left frontal pole (r=0.572). (l) Left middle temporal gyrus (r=0.488). Color image is available online at www.liebertpub.com/neu
FIG. 2.
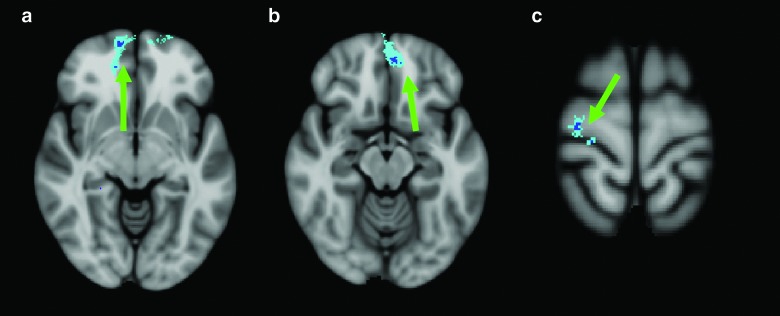
Images are displayed right on left, using the Harvard-Oxford Cortical Structural Atlas from FSL 4.1.3 for reference. (a)–(c) demonstrate significant regions (dark blue false discovery rate corrected region surrounded by additional Teal cluster analysis, indicated by green arrow) associated with a negative correlation between slow-wave root mean square amplitude and performance on Delis-Kaplan Executive Function System (D-KEFS) Trail Making Number Letter Switching Scaled (i.e., poorer performance on task with higher slow-wave amplitudes). (a) Right frontal pole (r=0.630). (b) Left frontal pole (r=0.546). (c) Right precentral gyrus (r=0.540). There were no positive correlations for this task. Color image is available online at www.liebertpub.com/neu
FIG. 3.

Images are displayed right on left, using the Harvard-Oxford Cortical Structural Atlas from FSL 4.1.3 for reference. (a) demonstrates a significant region (dark blue false discovery rate (FDR) corrected region surrounded by additional Teal cluster analysis, indicated by green arrow) associated with a negative correlation between slow-wave root mean square (RMS) amplitude and performance on Wechsler Adult Intelligence Scale (WAIS) Digit Symbol Coding Scaled (i.e., poorer performance on task with higher slow-wave amplitudes). (a) Right middle temporal gyrus/inferior temporal gyrus (r=0.568). (b)–(d) demonstrate significant regions (red FDR corrected region surrounded by additional yellow cluster analysis, indicated by green arrow) associated with a positive correlation between slow-wave RMS amplitude and performance on WAIS Digit Symbol Coding Scaled (i.e., better performance on tasks with a higher slow-wave amplitude). (b) Left superior parietal lobe/postcentral gyrus (r=0.556). (c) Right precentral gyrus (r=0.577). (d) Left frontal pole (r=0.540). Color image is available online at www.liebertpub.com/neu
Figure 1a–i demonstrates regions associated with a significant negative correlation (r value) between slow-wave RMS amplitude and performance on D-KEFS Color Word Interference Inhibition scaled score (i.e., poorer performance on task with higher slow-wave amplitudes). Figure 1a–c demonstrate significant negative correlations found in the frontal lobes with Figure 1a displaying the right superior/middle frontal gyrus (r=0.511), Figure 1b displaying the right frontal pole (r=0.462), and Figure 1c displaying the right anterior cingulate gyrus (r=0.472). Figure 1d shows significant negative correlations in the bilateral inferior parietal lobe (parietal operculum and supramarginal gyrus) with a left r value of 0.570 and a right r value of 0.520. Figure 1e demonstrates negative correlations found in the bilateral planum temporale and Heschl's gyrus, including H1 and H2, with a left r value of 0.475 and a right r value of 0.567. Figure 1f displays the negative correlation in the right precuneous cortex and cingulate gyrus (r=0.547). Figure 1g displays right-side negative correlation in the insular cortex (r=0.492). Figure 1h demonstrates significant negative correlations in the bilateral hippocampus and parahippocampal gyrus (left, r=0.452; right, r=0.489). The final negative correlation was found in the left temporal fusiform/inferior temporal gyrus (r=0.507) and is shown in Figure 1i.
Figure 1j–l demonstrate regions associated with a significant positive correlation between slow-wave RMS amplitude and performance on D-KEFS Color-Word Interference Inhibition scaled score (i.e., better performance on tasks with a higher slow-wave amplitude), an unexpected finding. Figure 1j shows the bilateral superior parietal lobes (left, r=0.452; right, r=0.489). Figure 1k demonstrates a small area of correlation in the left frontal pole (r=0.572) and, finally, Figure 1l shows a small area of correlation found in the left middle temporal gyrus (r=0.488).
Displayed in Figure 2a–c are statistically significant regions associated with a negative correlation between slow-wave RMS amplitude and performance on D-KEFS Trail Making Number Letter Switching scaled score. Figure 2a shows correlation in the right frontal pole (r=0.630. Figure 2B displays a cluster in the left frontal pole (r=0.546) and, finally, Figure 2c demonstrates a cluster in the right precentral gyrus (r=0.540). There are no significant clusters with a positive correlation between slow-wave RMS amplitude and neuropsychological testing performance for this task.
Displayed in Figure 3a, a small, yet significant, region associated with a negative correlation between slow-wave RMS amplitude and performance on WAIS Digit Symbol Coding scaled score is shown in the right middle temporal gyrus/inferior temporal gyrus (r=0.568. Figure 3b–d demonstrates regions associated with a significant positive correlation between slow-wave RMS amplitude and performance on WAIS Digit Symbol Coding scaled score. Figure 3b indicates a significant cluster found in the left superior parietal lobe/postcentral gyrus (r=0.556). Figure 3c demonstrates a significant cluster found in the right precentral gyrus (r=0.577) and, finally, Figure 3d shows a significant cluster in the left frontal pole (r=0.540).
Discussion
In the present study, our results indicate significant cognitive differences between participants exhibiting persistent PCS post-mTBI and HCs. In addition, though utilizing an objective approach, we were able to correlate abnormal MEG slow-wave activity to performance on the significant cognitive tests. The fundamental importance of this study is the results of the correlations between cognitive performance measures and MEG slow-wave imaging results that help to provide evidence of the neurobiological underpinnings of mTBI with lasting PCS. Results of this study were consistent with the previous neuropsychological studies and the integrated neuropsychological and imaging approach utilized in the Lewine and colleagues study.14–16,28 These results support our hypotheses that neuropsychological differences would be found between a group with lasting PCS post-mTBI and that significant correlations would be found between neuropsychological measures that display significance between the two groups and MEG slow-wave activity relational to specific neural coordinates associated with the neuropsychological measures utilized.
Despite a multitude of studies examining clinical outcomes post-mTBI, there is continued controversy about the underlying cause of persistent symptoms. Researchers have suggested that noninjury variables (i.e., psychiatric history, emotional status, gender, and motivational factors) may contribute to the development of long-term symptoms and disability post-mTBI. The diagnosis of mTBI is, by definition, associated with normal results on clinical neuroimaging studies; the resulting lack of a biological gold standard to reliably diagnose mTBI has contributed to diagnostic confusion and to the perception that persistent symptoms post-mTBI are unrelated to changes in brain structure or function. To further complicate controversy over origins of persistent symptoms, previous studies of mTBI have used variable diagnostic criteria and many have serious methodological limitations (e.g., small sample size, lack of control groups, biased sampling, and variability in the time postinjury).
Recently, researchers have observed subtle electrophysiological abnormalities in mTBI patients on measures of resting state slow-wave activity using MEG. These studies provide some of the most objective evidence that mTBI contributes to changes in brain function.28,30 The current study further characterizes abnormalities in slow-wave activity post-mTBI in a group of patients with symptoms lasting 3 months or longer. We examined the relationship between performance on a standardized battery of cognitive tests and abnormalities in slow-wave activity as measured by MEG using data processing techniques recently developed in our lab. The results from both the neurocognitive and MEG evaluations were also compared to those of healthy matched controls to determine the pattern and level of functioning in the mTBI+PCS group.
A battery of standardized and validated neuropsychological tests was selected to assess a range of cognitive abilities, changes in which have been associated with TBI, including learning and memory, attention, executive functioning, and motor speed.39 A MANCOVA comparing group performance on the cognitive battery revealed that the mTBI+PCS group performed worse overall than the HC group, even when differences in anxiety and premorbid verbal functioning were taken into consideration. Follow-up ANCOVAs revealed that the mTBI+PCS group performed worse, specifically on complex timed measures of cognitive flexibility, inhibition, working memory, and initiation, in addition to processing speed. These tests are highly sensitive to injury to the frontal and subcortical regions of the brain, particularly in the dorsolateral frontal cortex. It should be noted that, despite group differences, the level of performance of the mTBI+PCS group was not within the clinically impaired range. However, these group differences are consistent with those documented in previous studies of mTBI neuropsychological impairments.16 Further, performance on these measures that is decreased from premorbid levels may help explain many of the principal symptom complaints from our mTBI+PCS group, such as mental fatigue, subjective slowness in cognitive processing, and difficulty multi-tasking, and may result in lowered functioning, particularly in challenging situations. D-KEFS subtests have been routinely utilized to assess various aspects of executive functioning, and because the test was designed to be challenging and involves speeded performance, it may have greater sensitivity to mild brain damage.67 The differences between our mTBI population and control population suggests that subtle deficits in processing speed, cognitive flexibility, or other higher order cognitive processes are associated with persistent symptoms post-mTBI.
It is important to note that there were no significant differences on tests of pure motor speed (D-KEFS Trailmaking Motor Speed, Grooved Pegboard), indicating that the differences on speeded tests were not owing to motor slowing. It is also important to note that there were no differences between the groups on a timed word reading task (D-KEFS Color-Word Interference Word Reading scaled score) nor any difference between groups on color naming (D-KEFS Color-Word Interference Color Naming scaled score), indicating that the differences were not owing to reading or scanning speed. In addition, groups were well matched on measures of general intellectual functioning, (i.e., the WAIS III VIQ and PIQ), suggesting that group differences did not result from global impairments or pre-existing intellectual differences. There were no significant group differences on tests of learning and memory, although there was a trend toward significance on the Short Delay Free Recall score on the CVLT.
After detection of significant differences between mTBI patients with persistent symptoms and HC participants on neuropsychological tasks, an additional aim of the study was identifying relationships between cognitive tests that showed group differences and regions of the brain that exhibited slow waves. Analyses showed significant FDR multiple comparison corrected correlations for variables from both the executive function tasks and the processing speed tasks with multiple areas of the brain. Further, an additional cluster analysis was also performed for correcting multiple comparisons across voxels. The size of the cluster for corrected p<0.05 was determined by the AlphaSim program in AFNI.61
Both executive function tasks that showed group differences, D-KEFS Color Word Interference Inhibition and Trail Making Number Letter Switching, had significant correlations with slow waves in the frontal lobes, specifically the right frontal pole. Whereas slow waves in the left frontal lobe correlated with the Trail Making Number Letter Switching variable, the left frontal lobe slow waves did not seem to correlate with poorer performance on the Color Word Interference task, suggesting that, for this task, right frontal lobe function is either fundamentally more important or that our population did not have as many slow waves in the left frontal lobe. It is possible that participants with semantic problems related to left frontal lobe dysfunction may actually perform better on Color-Word Interference, owing to a reduction in interference. In addition, Risse and collegues, in a study of frontal lobe epilepsy, showed that postoperative decline on a measure of cognitive flexibility was greatest in patients who underwent resections of the right frontal pole and prefrontal cortex, rather than left.68
Our findings may also reflect dysfunction in the frontoparietal executive function network, which includes prefrontal regions, such as the frontopolar cortex, the prefrontal cortex, the dorsolateral prefrontal cortex, the anterior cingulate, and the inferior parietal lobe, and often engages in tasks that require executive control.69 Recruitment of the frontoparietal network is critical for goal-directed higher-level cognition processes involving integration and control. As a source of top-down control in the brain, the frontoparietal network and its connectivity pattern provides an architecture for executive function. Damage to these key areas may contribute to poorer control and integration. In our study, the MEG correlations with performance on D-KEFS Color Word Interference Inhibition show that poorer performance on this executive function task correlates with slow-wave activity in areas in the frontoparietal network. This correlation in mTBI participants with lasting symptoms provides further support that cognitive differences from controls are related to underlying neuropathology rather than, or in addition to, psychiatric factors or motivational, secondary gain issues.
The correlation between poorer performance on the D-KEFS Color-Word Interference Inhibition trial and slow waves in the right anterior cingulate cortex (ACC) is also interesting. Because the ACC maintains reciprocal interconnections with the lateral prefrontal cortex, which can include the frontal pole, slow waves in the ACC may impair performance on executive functioning tasks.70 Because the ACC helps to facilitate impulse control and maintain attention, dysfunction in this area reflected by slow waves would allow for increased errors as well as longer times for task performance.
The posterior parietal lobe, with its extensive connections to temporal and frontal lobes, has been long recognized as a neural substrate for attention, particularly visuospatial attention.71–73 There is a growing consensus that attention and executive control are inter-related and share neural substrates.74,75 In a recent study, cognitive executive control and functioning was found to be associated with the white matter underlying the supramarginal gyrus, a region identified in the present study as exhibiting slow waves.73 As our previous study in 2009 demonstrated that regions of white matter with reduction in intensity can link to the nearby slow-wave generating gray matter, there is a distinct possibility that slow waves generated in this area, and quite possibly other regions, that are correlating to poorer neurocognitive scores may be related to reduced anisotropy in the neighboring white matter region.57
Although the study demonstrates relationships between affected regions of the brain and cognitive tests, it is important to note that we are not suggesting one-to-one correspondence between brain areas displaying slow waves and direct test measures. Given the complexity of the brain, there may be brain regions demonstrating slow waves previously unknown to affect test measures that correlate with poor participant performance on cognitive measures. Additionally, though we did our best to draw from a wide variety of different testing measures, certain brain regions showing slow waves may not negatively impact performance on the testing measures chosen (see positive correlations noted in our data). Owing to differing functional connectivities of many brain regions, there are corresponding multi-functionalities of the brain that remain unexplored. As demonstrated in the Huang and colleagues article, whereas certain gray matter areas exhibiting slow waves linked to nearby injured fiber tracts with reduced fractional anisotropy (FA) detected using DTI, a different pattern of slow-waves emerged when reduced FA was detected in a major fiber tract.57 When a major fiber tract was found to have reduced FA, slow-wave–generating areas were not found to be directly adjacent to the area where the reduced FA was observed. This may be the case for some of the observed effects in this study.
Recent interest has focused on potential long-term consequences of mTBI and concussion, such as depression and cognitive changes occurring years after sport-related TBIs or increased risk of dementia in individuals with previous TBI.23,76 Given that it is unclear why some persons rapidly recover and others may exhibit long-term cognitive decline later in life, one hypothesized mechanism is a decrease in cognitive reserve at a faster rate, leaving individuals more vulnerable to age-related changes or other factors that increase risk for depression and mild cognitive impairment.77,78 Whereas longitudinal studies are needed to control for other factors that influence long-term cognitive health, our study begins to identify neurobiological underpinnings of mTBI that may explain why certain individuals may be more affected than others after a concussion or series of concussions. In addition, MEG may ultimately provide a means for detecting TBI-related increased risk for further cognitive and psychiatric changes and for targeting prevention interventions.
The primary limitation of this study was the lack of active duty military controls, although the control group did include retired or separated military personal. A strong effort was made to keep the groups as demographically similar as possible, and covariates in the analysis were utilized to ensure any differences would be accounted for. Future studies should include active duty military personnel without any exposure to blast or TBI; currently, data from our groups' ongoing studies with uninjured military personnel are being analyzed and can be included in future reports.
An additional limitation is that abnormal delta slow-wave activity is not pathognomonic to TBI and can be present in other neurological disorders, such as stroke, epilepsy, and brain tumors. To control for this, participants in the study were carefully screened for previous neurological disorders and each MRI was read by a board-certified radiologist. In addition, certain medications are known to increase global delta slow-wave power (e.g., neuroleptic sedatives, antidepressants, and hypnotics; participants taking these medications were excluded from the study or were required to be off of the medications for three half-lives before the study sessions).35 Owing to the potential clinical applications, and the reality that exclusions for those on medications may not be realistic in a clinical setting, future studies need to examine the effect of medications and whether they may be controlled by utilizing different analysis strategies.
These results provide objective evidence of lasting neurophysiological changes in brain function post-mTBI. This study utilized rigorous inclusion criteria, including reading of any neuroimaging studies of all participants to ensure that there were no previously undiagnosed, clinically significant lesions in any participants, including controls. Further, once enrolled in the study, all participants underwent standardized assessment of effort, to reduce potential confounds related to motivational factors. Results of this study were consistent with findings from a number of previous studies of mTBI and support our hypothesis that mTBI can contribute to persistent PCS and that subtle neurophysiological changes observed using MEG may provide a biological marker to improve diagnostic accuracy of mTBI.14–16,28 Finally, these results could be used to develop evidence-based treatment guidelines to address the subtle functional declines that occur in some individuals after a single uncomplicated mTBI. Future studies will need to include subjects with mTBI without PCS in order to further understand the effects of abnormal slow-wave activity on MEG. Future directions include adding additional modalities, such as DTI, to detect correlations in white matter FA with gray matter slow-wave MEG correlates as well as investigating individuals with mTBI and lasting PCS utilizing a longitudinal approach to detect the neural coordinates that correlate with loss of cognitive reserve over time, while controlling for outside variables that may have an impact on cognitive decline.
Acknowledgments
This work was supported by Merit Review Grants from the Department of Veterans Affairs (to M.X.H.; I01-CX000499, NURC-022-10F, and NEUC-044-06S). The authors thank Dr. Rebecca Theilmann for her efforts in preparing the MRI protocol and Terry Curry (RN) for his effort in recruiting TBI participants. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC), National Center for Injury Prevention and Control. (2003). Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Centers for Disease Control and Prevention: Atlanta, GA [Google Scholar]
- 3.Ruff R.M. (2011). Mild traumatic brain injury and neural recovery: rethinking the debate. NeuroRehabilitation 28, 167–180 [DOI] [PubMed] [Google Scholar]
- 4.Dolan S., Martindale S., Robinson J., Kimbrel N., Meyer E.C., Kruse M.I., Morissette S.B., Young K.A., and Gulliver S. (2012). Neuropsychological sequelae of PTSD and TBI following war deployment among OEF/OIF veterans. Neuropsychol. Rev. 22, 21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belanger H.G., Uomoto J.M., and Vanderploeg R.D. (2009). The Veteran's Health Administrations (VHA's) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J. Head Trauma Rehabil. 24, 4–13 [DOI] [PubMed] [Google Scholar]
- 6.Levin H.S., Mattis S., Ruff R.M., Eisenberg H.M., Marshall L.F., Tabaddor K., High W.M., and Frankowski R.F. (1987). Neurobehavioral outcome following minor head injury: a three-center study. J. Neurosurg. 66, 234–243 [DOI] [PubMed] [Google Scholar]
- 7.Binder L.M., Rohling M.L., and Larrabee G.J. (1997). A review of mild head trauma. Part I: meta-analytic review of neuropsychological studies. J. Clin. Exp. Neuropsychol. 19, 421–431 [DOI] [PubMed] [Google Scholar]
- 8.Frencham K.A., Fox A.M., and Maybery M.T. (2005). Neuropsychologicl studies of traumatic brain injury:a meta-analytic review of research since 1995. J. Clin. Exp. Neuropsychol. 27, 334–351 [DOI] [PubMed] [Google Scholar]
- 9.Rohling M.L., Binder L.M., Demakia G.J., Ploetz D.M., and Langhinrichsen-Rohling J. (2011). A meta-analysis of neuropsychological outcome after mild traumatic brain injury: re-analyses and reconsiderations of Binder et al. (1997), Frencham et al. (2005) and Pertab et al. (2009). Clin. Neuropsychol. 25, 608–623 [DOI] [PubMed] [Google Scholar]
- 10.Powell T.J., Collin C., and Sutton K. (1996). A follow-up study of patients hospitalized after minor head injury. Disabil. Rehabil. 18, 231–237 [DOI] [PubMed] [Google Scholar]
- 11.Deb S., Lyons I., and Koutzoukis C. (1999). Neurobehavioural symptoms one year after a head injury. Br. J. Psychiatry 174, 360–365 [DOI] [PubMed] [Google Scholar]
- 12.Pertab J.L., James K.M., and Bigler E.D. (2009). Limitations of mild traumatic brain injury meta-analyses. Brain Inj. 23, 498–508 [DOI] [PubMed] [Google Scholar]
- 13.Bigler E.D., Farrer T.J., Pertab J.L., James K., Petrie J.A., and Hedges D.W. (2013). Reaffirmed limitations of meta-analytic methods in the study of mild traumatic brain injury: a response to Rohling et al. Clin. Neuropsychol. 27, 176–214 [DOI] [PubMed] [Google Scholar]
- 14.Vanderploeg R.D., Curtis G., and Belanger H.G. (2005). Long-term neuropsychological outcomes following mild traumatic brain injury. J. Int. Neuropsychol. Soc. 11, 228–236 [DOI] [PubMed] [Google Scholar]
- 15.Sterr A., Herron K.A., Hayward C., and Montaldi D. (2006). Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. BMC Neurol. 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson B., Berglund P., and Ronnback L. (2009). Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj. 23, 1027–1040 [DOI] [PubMed] [Google Scholar]
- 17.Guskiewicz K.M., Marshall S.W., Broglio S.P., Cantu R.C., and Kirkendall D.T. (2002). No evidence of impaired neurocognitive performance in collegiate soccer players. Am. J. Sports Med. 30, 157–162 [DOI] [PubMed] [Google Scholar]
- 18.Broglio S.P., Pontifex M.B., O'Connor P., and Hillman C.H. (2009). The persistent effects of concussion on neuroelectric indices of attention. J. Neurotrauma, 26, 1463–1470 [DOI] [PubMed] [Google Scholar]
- 19.Tsanadis J., Montoya E., Hanks R.A., Millis S.R., Fichtenberg N.L., and Axelrod B.N. (2008). Brain injury severity, litigation status, and self-report of postconcussive symptoms. Clin. Neuropsychol. 22, 1080–1092 [DOI] [PubMed] [Google Scholar]
- 20.Lishman W.A. (1998). Organic Psychiatry (3rd ed.). Blackwell Science: London [Google Scholar]
- 21.Bryant R.A., and Harvey A.G. (1999). Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. J. Nerv. Ment. Dis. 187, 302–305 [DOI] [PubMed] [Google Scholar]
- 22.Bigler E.D. (2001). The lesion(s) in traumatic brain injury: implications for clinical neuropsychology. Arch. Clin. Neuropsychol. 16, 95–131 [PubMed] [Google Scholar]
- 23.Lees-Haley P.R., Fox D.D., and Courtney J.C. (2001). A comparison of complaints by mild brain injury claimants and other claimants describing subjective experiences immediately following their injury. Arch. Clin. Neuropsychol. 16, 689–695 [PubMed] [Google Scholar]
- 24.Lees-Haley P.R., Green P., Rohling M.L., Fox D.D., and Allen L.M., III (2003). The lesion(s) in traumatic brain injury: implications for clinical neuropsychology [commentary]. Arch. Clin. Neuropsychol. 18, 585–594 [PubMed] [Google Scholar]
- 25.Landre N., Poppe C.J., Davis N., Schmus B., and Hobbs S.E. (2006). Cognitive functioning and postconcussive symptoms in trauma patients with and without mild TBI. Arch. Clin. Neuropsychol, 21 255–273 [DOI] [PubMed] [Google Scholar]
- 26.van der Naalt J., van Zomeren A.H., Sluiter W.J., and Minderhoud J.M. (1999). One year outcome in mild to moderate head injury: the predictive value of acute injury characteristics related to complaints and return to work. J. Neurol. Neurosurg. Psychiatry 66, 207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dikmen S., Machamer J., Fann J.R., and Temkin N.R. (2010). Rates of symptom reporting following traumatic brain injury. J. Int. Neuropsychol. Soc. 16, 401–411 [DOI] [PubMed] [Google Scholar]
- 28.Lewine J.D., Davis J.T., Bigler E.D., Thoma R., Hill D., Funke M., Sloan J.H., Hall S., and Orrison W.W. (2007). Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J. Head Trauma Rehabil. 22, 141–155 [DOI] [PubMed] [Google Scholar]
- 29.Huang M.X., Huang C.W., Robb A., Angeles A., Nichols S.L., Baker D.G., Song T., Harrington D.L., Theilmann R.J., Srinivasan R., Heister D., Diwakar M., Canive J.M., Edgar J.C., Chen Y.H., Shen M., El-Gabalawy F., Levy M., McLay R., Webb-Murphy J., Liu T.T., Drake A., and Lee R.R. (2014). MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images. Neuroimage 84, 585–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang M.X., Nichols S., Robb A., Angeles A., Drake A., Holland M., Asmussen S., D'Andrea J., Chun. W., Levy M., Cui L., Song T., Baker D.G., Hammer P., McLay R., Theilmann R.J., Coimbra R., Diwakar M., Boyd C., Neff J., Liu T.T., Webb-Murphy J., Farinpour R., Cheung C., Harrington D.L., Heister D., and Lee R.R. (2012). An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. Neuroimage 61, 1067–1082 [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. (2001). Wechsler Test of Adult Reading. The Psychological Corporation: San Antonio, TX [Google Scholar]
- 32.Morey L.C. (2005). Personality Assessment Inventory (PAI) Software Portfolio. Psychological Assessment Resources, Inc.: Lutz, FL [Google Scholar]
- 33.ACRM; Mild Traumatic Brain Injury Committee. (1993). Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 8, 86–87 [Google Scholar]
- 34.Teasdale G., and Jennett B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 35.Niedermeyer E., and Lopes da Silva F. (2005). Electroencephalography: Basic Principles, Clinical Applications, and Related Fields (5th ed.) Lippincott Williams & Wilkins: Philidelphia, PA [Google Scholar]
- 36.Tombaugh T.N. (1996). Test of Memory Malingering. MultiHealth Systems: Toronto, Ontario, Canada [Google Scholar]
- 37.Bianchini K.J., Curtis KL, and Greve KW. (2006). Compensation and malingering in traumatic brain injury: a dose-response relationship? Clin. Neuropsychol. 20, 831–847 [DOI] [PubMed] [Google Scholar]
- 38.Davis J.J., McHugh T.S., Axelrod B.N., and Hanks R.A. (2012). Performance validity and neuropsychological outcomes in litigants and disability claimants. Clin. Neuropsychol. 26, 850–865 [DOI] [PubMed] [Google Scholar]
- 39.Bagiella E., Novack T.A., Ansel B., Diaz-Arrastia R., Dikmen S., Hart T., and Temken N. (2010). Measuring outcome in traumatic brain injury treatment trials: recommendations from the traumatic brain injury clinical trials network. J. Head Trauma Rehabil. 25, 375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delis D.C., Kramer J., Kaplan E., and Ober B.A. (2000). The California Verbal Learning Test-2. The Psychological Corporation: San Antonio, TX [Google Scholar]
- 41.Benedict R. (1997). Brief Visuospatial Memory Test-Revised. Psychological Assessment Resources, Inc.: Lutz, FL [Google Scholar]
- 42.Wechsler D. (1997). Wechsler Adult Intelligence Scale, Third Edition The Psychological Corporation: San Antonio, TX [Google Scholar]
- 43.Conners K. (2004). Conners' Continuous Performance Test II. Multi-Health Systems, Inc.: North Tonawanda, NY [Google Scholar]
- 44.Delis D.C., Kaplan E., and Kramer J.H. (2001). Delis-Kaplan Executive Function System. The Psychological Corporation: San Antonio, TX [Google Scholar]
- 45.Trites R.L. (1977). Neuropsychological Test Manual. Royal Ottawa Hospital: Ottawa, Ontario, Canada [Google Scholar]
- 46.Heaton R.K., Walden Miller S., Taylor M.J., and Grant I. (2004). Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults, Professional Manual. Psychological Assessment Resources, Inc.: Lutz, FL [Google Scholar]
- 47.Cramer E.M., and Bock R.D. (1966). Multivariate Analysis. Rev. Educ. Res. 36, 604–617 [Google Scholar]
- 48.Meyers L.S., Gamst G., and Guarino A. (2006). Applied Multivariate Research: Design and Interpretation. Sage: Thousand Oaks, CA [Google Scholar]
- 49.Benjamini Y., and Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Statist. Soc. 57, 289–300 [Google Scholar]
- 50.Cohen D., Schläpfer U., Ahlfors S., Hämäläinen M., and Halgren E. (2002). New Six-Layer Magnetically-Shielded Room for MEG. Proceedings of Biomag, 2002. Available at: http://www.nmr.mgh.harvard.edu/meg/pdfs/2002BiomagProceed%28ourMSR%29.pdf Accessed May31, 2015 [Google Scholar]
- 51.Taulu S., Kajola M., and Simola J. (2004). Suppression of interface and artifacts by the signal space separation method. Brain Topogr. 16, 269–275 [DOI] [PubMed] [Google Scholar]
- 52.Taulu S., Simola J., and Kajola M. (2004). MEG recordings of DC fields using the signal space separation method (SSS). Neurol. Clin. Neurophysiol. 2004, 35. [PubMed] [Google Scholar]
- 53.Song T., Gaa K., Cui L., Feffer L., Lee R.R., and Huang MX. (2008). Evaluation of signal space separation via simulation. Med. Biol. Eng. Comput. 46, 923–932 [DOI] [PubMed] [Google Scholar]
- 54.Song T., Cui L., Gaa K., Feffer L., Taulu S., Lee R.R., and Huang MX. (2009). Signal space separation algorithm and its application on suppressing artifacts caused by vagus nerve stimulation for magnetoencephalography recordings. J. Clin. Neurophysiol. 26, 392–400 [DOI] [PubMed] [Google Scholar]
- 55.Mosher J.C., Leahy R.M., and Lewis P.S. (1999). EEG and MEG: forward solutions for inverse methods. IEEE Trans. Biomed. Eng. 46, 245–259 [DOI] [PubMed] [Google Scholar]
- 56.Huang M.X., Song T., Hagler D.J., Jr., Podgorny I., Jousmaki V., Cui L., Gaa K., Harrington D.L., Dale A.M., Lee R.R., Elman J., and Halgren E. (2007). A novel integrated MEG and EEG analysis method for dipolar sources. Neuroimage 37, 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang M.X., Theilmann R.J., Robb A., Angeles A. N.ichols S., Drake A., D'Andrea J., Levy M., Holland M., Song T., Ge S., Hwang E., Yoo K., Baker D.G., Trauner D., Coimbra R., and Lee R.R. (2009). Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma 26, 1213–1226 [DOI] [PubMed] [Google Scholar]
- 58.Huang MX., Dale A.M., Song T., Halgren E., Harrington D.L., Podorny I., Canive J.M., Lewis S., and Lee R.R. (2006). Vector-based spatial-temporal minimum L1-norm solution for MEG. Neuroimage 31, 1025–1037 [DOI] [PubMed] [Google Scholar]
- 59.Jenkinson M., and Smith S.M. (2001). A global optimization method for robust affine registration of brain images. Med. Image Anal. 5, 143–156 [DOI] [PubMed] [Google Scholar]
- 60.Jenkinson M., Bannister P.R., Brady J.M., and Smith S.M. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 [DOI] [PubMed] [Google Scholar]
- 61.Ward B.D. (2000). Simultaneous interference for fMRI data. AFNI 3dDeconvolve documentation. Medical College of Wisconsin: Milwaukee, WI [Google Scholar]
- 62.Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., and Smith S.M. (2012). FSL. Neuroimage 62, 782–790 [DOI] [PubMed] [Google Scholar]
- 63.Makris N., Goldstein J.M., Kennedy D., Hodge S.M., Caviness V.S., Faraone S.V., Tsuang M.T., and Seidman L.J. (2006). Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr. Res. 83, 155–171 [DOI] [PubMed] [Google Scholar]
- 64.Frazier J.A., Chiu S., Breeze J.L., Makris N., Lange N., Kennedy D.N., Herbert M.R., Bent E.K., Koneru V.K., Dieterich M.E., Hodge S.M., Rauch S.L., Grant P.E., Cohen B.M., Seidman L.J., Caviness V.S., and Biedermann J. (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am. J. Psychiatry 162, 1256–1265 [DOI] [PubMed] [Google Scholar]
- 65.Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., and Killiany R.J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 [DOI] [PubMed] [Google Scholar]
- 66.Goldstein J.M., Seidman L.J., Makris N., Ahern T., O'Brien L.M., Caviness V.S., Jr., Kennedy D.N., Faraone S.V., and Tsuang M.T. (2007). Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol. Psychiatry 61, 935–945 [DOI] [PubMed] [Google Scholar]
- 67.Homack S., Lee D., and Riccio C.A. (2005). Test review: Delis-Kaplan executive function system. J. Clin. Exp. Neuropsychol. 27, 599–609 [DOI] [PubMed] [Google Scholar]
- 68.Risse G.L. (2006). Cognitive outcome in patients with frontal lobe epilepsy. Epilepsia 47, Suppl. 2, 87–89 [DOI] [PubMed] [Google Scholar]
- 69.Barbey A.K., Colom R., Solomon J., Kruegar F., Forbes C., and Grafman J. (2012). An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain 135, Pt. 4, 1154–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bush G., Luu P., and Posner M.I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222 [DOI] [PubMed] [Google Scholar]
- 71.Saalmann Y.B., Pigarev I.N., and Vidyasagar T.R. (2007). Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science 316, 1612–1615 [DOI] [PubMed] [Google Scholar]
- 72.Bushman T.J., and Miller E.K. (2007). Top down-versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315, 1860–1862 [DOI] [PubMed] [Google Scholar]
- 73.Yin X., Zhao L., Junhai X., Evans A.C., Fan L., Ge. H., Tang Y., Khundrakpam B., Wang J., and Liu S. (2012). Anatomical substrates of the alerting, orienting and executive control components of attention: focus on the posterior parietal lobe. PLoS One 7, e50590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fan J., McCandlis B.D., Fossella J., Flombaum J.I., and Posner M.I. (2005). The activation of attentional networks. Neuroimage 26, 471–479 [DOI] [PubMed] [Google Scholar]
- 75.Posner M.I., and Rothbart M.K. (2007). Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 58, 1–23 [DOI] [PubMed] [Google Scholar]
- 76.Broglio S.P., Ferrara M.S., Piland S.G., and Anderson R.B. (2006). Concussion history is not a predictor of computerized neurocognitive performance. Br. J. Sports Med. 40, 802–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Broglio S.P., Eckner J.T., Paulson H.L., and Kutcher J.S. (2012). Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc. Sport Sci. Rev. 40, 138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stern Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsycholo. 8, 448–460 [PubMed] [Google Scholar]