Abstract
Objective:
Synovial sarcoma (SS) of the head and neck is an unusual malignancy. This article documents five SSs in this region.
Methods:
All the patients underwent MR examinations. Four lesions received surgical ablation; one was treated with radiotherapy before surgery. The clinical, pathological and MRI features were reviewed.
Results:
Four of all five cases were monophasic fibrous-type SS, and the other one was biphasic type that was the fourth documented SS located in the nasopharynx. The symptoms were varied. All the masses were well defined, mainly homogeneous and solid; three of them arose adjacent to the minor joint. The mass parenchyma showed isointense signal on T1 weighted imaging similar to that of the skeletal muscle and hyperintense signal on T2 weighted imaging with remarkable enhancement. Two cases were found with fibrous septum, one with haemorrhage and one with cystic degeneration. Epithelial membrane antigens (EMAs) were all positive. The positive rate of cytokeratin (CK), part pan-CK antibody (AE1/3) and vimentin (Vim) were 50%, 75%, 75%, respectively.
Conclusion:
Well-defined head and neck masses frequently arise adjacent to the minor joint, which are mainly homogeneous and solid, with isointense signal on T1 weighted MRI and hyperintense signal on T2 weighted MRI, and remarkable enhancement should evoke the diagnosis of SS. The positive staining of Vim\AE1/3\EMA and CK facilitates the final diagnosis.
Advances in knowledge:
The article documents the fourth SS involving the nasopharynx; other locations were also uncommon; three of them arose adjacent to the minor joint. The clinical, pathology and uncommon MR features of SS in the head and neck are also documented.
The first documented head and neck synovial sarcoma (SS) was described by Jernstrom1 in 1954, which involved the pharynx. Owing to the tumour's histological manifestation similar to synovium, SS was thought to derive from a synovial membrane in the past. In fact, SS does not originate from mature synovial cells. It has been recently classified as a tumour of uncertain histogenesis.2
Only 3–10% of SSs arise in the head and neck region.3,4 Many cases of head and neck SS have been reported, from the skull base to the hypopharynx. The radiological features of SS have been well documented in literature.3,5,6 A heterogeneous mass with “triple sign” [hypo-, iso- and hyperintense signal on T2 weighted MRI (T2WI)] that represents the existence of different tissue such as fibrous tissue, haemorrhage, cystic degeneration and “fluid-fluid level”; and calcifications on plain CT and radiographs are also important features of SS. Little research has focused on the uncommon radiological findings. This article documents unusual MR manifestations of five SSs in the head and neck region. The clinical findings and pathology are also illustrated.
METHODS AND MATERIALS
With approval of all protocols from the institutional review board of the Fudan University Shanghai Cancer Center, five pathology-proven SSs of the head and neck region during a 5-year period (January 2005–09) were retrospectively reviewed. Informed consent was acquired from every participant before the imaging studies. Complete details of clinical findings, pathological and MR features of all patients and immunohistochemistry (IHC) results of four patients were collected for analysis.
All the patients underwent MRI examinations with a 1.5-T superconducting MR system (Signa Twin Speed Exite, GE Medical Systems, Millwaukee, WI), with 4- to 5-mm slice thickness and 0.5- to 2.0-mm gap spacing. Axial T1 weighted MRI (T1WI) with or without fat saturation was performed before and after the injection of contrast medium, and axial and coronal T2WI were also performed. Gadolinium-diethylene triamine pentaacetic acid (Gd-DTPA) (Bayer Schering Pharma AG, Berlin Germany) was used as the contrast agent at a dose of 0.1 mmol kg−1 and intravenous infuse rate of 2 ml s−1. Plain CT or radiograph of the chest was performed for pulmonary scanning. One patient underwent both plain and enhanced CT scans for the primary lesion site.
All the lesions were independently evaluated by two radiologists, regarding location, size, shape, internal architecture, homogeneity, margin definition, MRI signal intensity (hypo-, iso- or hyperintense signal) and pattern of enhancement for all tumours. Results were recorded by consensus. The enhancements of the lesions were assessed subjectively: mild enhancement was defined as enhancement resembling that of the adjacent muscle; moderate enhancement was defined as enhancement more obvious than that of the muscle; and remarkable enhancement was defined as enhancement resembling that of the adjacent vessels.
The inspection reports of light microscope of all patients and IHC data of four lesions were collected.
RESULTS
Clinical and radiological findings
Three females and two males were included in this study, with an average age of 26 years and ranging from 12 to 38 years. All patients were either adolescents or young adults. The manifestations of SS were varied with location of the mass. Two cases presented with local slow-growth masses without pain; one case presented with weakness of the upper limbs; one manifested as difficulty with mouth opening; and the last one complained about epistaxis (Table 1).
Table 1.
Clinical findings and pathological types of synovial sarcoma
| Number | Age (years) | Gender | Presentation | Location | Pathology |
|---|---|---|---|---|---|
| Case 1 | 12 | F | Weakness of the left upper limb | Left paravertebral space near zygapophysial joints | Monophasic |
| Case 2 | 26 | F | Local mass | Right submaxillary space | Monophasic |
| Case 3 | 38 | M | Difficulty of opening the mouth | Right temporomandibular space around temporomandibular joint | Monophasic |
| Case 4 | 24 | M | Local mass | Retrovetebral space adjacent atlantooccipital joint | Monophasic |
| Case 5 | 30 | F | Epistaxis | Right pharynx nasalis | Biphasic |
F, female; M, male.
The lesions ranged in size from 4.0 to 7.5 cm in diameter. The locations of the lesions were in the paravertebral space near the vertebral minor joint (zygapophysial joints), the submaxillary space, the retroveterbral space adjacent to the atlantooccipital joint, pharynx nasalis and the temporomandibular space around the temporomandibular joint. One patient was found with the enlargement of the cervical 6/7 intervertebral foramen, with invasion to the spinal cord and the apex of the left lung. Three masses were located adjacent to the minor joints in the head and neck.
All the masses were well defined, mainly homogeneous and solid, and sometimes lobulated. On MRI, the parenchyma of lesions showed as isointense signal similar to the skeletal muscle on unenhanced T1WI, hyperintense signal on T2WI and remarkable enhancement post Gd-DTPA injection (Figures 1–5). Fibrous septum was observed in two lesions (n = 2), which manifested as hypointense signals on both T1WI and T2WI with mild enhancement on the contrast scan (Figures 2 and 4). Haemorrhage was observed in one mass (n = 1) (Figure 3a), which manifested as high signal intensity on both unenhanced T1WI and T2WI without enhancement. Cystic degeneration was observed in one case (n = 1) (Figure 3b–d), which manifested as hyposignal intensity on T1WI and as high signal intensity on T2WI with no enhancement. None of the masses demonstrated “fluid-fluid level”. Only one patient (Table 1, Case 4) underwent CT scans. A homogeneous mass with notable enhancement was revealed without apparent calcification.
Figure 1.
Synovial sarcoma in the left paravertebral space of a 12-year-old girl. (a) Axial T1 weighted MR image shows a well-defined homogeneous mass with isosignal intensity similar to the muscle. (b) Axial T2 weighted MR image shows homogeneous lobulated mass involving via left C6/7 intervertebral foramen into the spinal canal with hypersignal intensity. (c) Coronal T2 weighted MR image shows enlargement of the left C6/7 intervertebral foramen, intraspinal and the apex of left lung involvement.
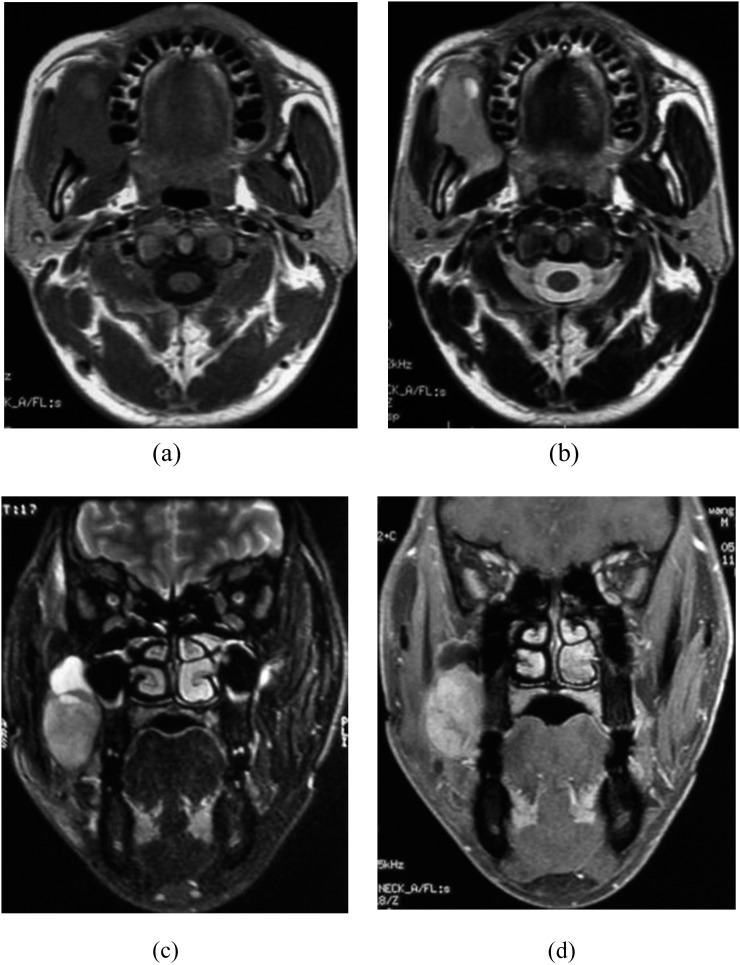
Figure 5.
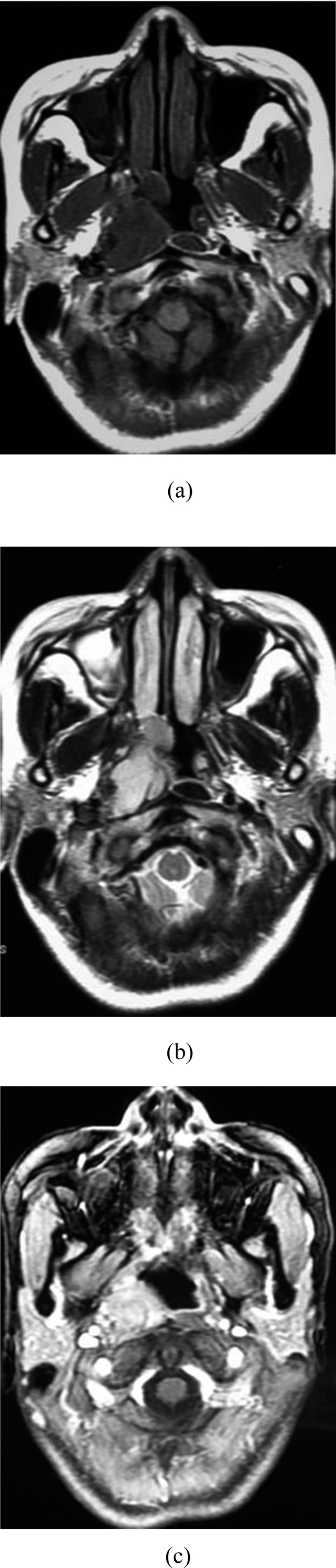
Synovial sarcoma (SS) in the right pharynx nasalis of a 30-year-old female. (a) Axial T1 weighted MRI of SS shows isosignal intensity. (b) Axial view shows hyper on T2 weighted MRI. (c) Axial view shows notable enhancement.
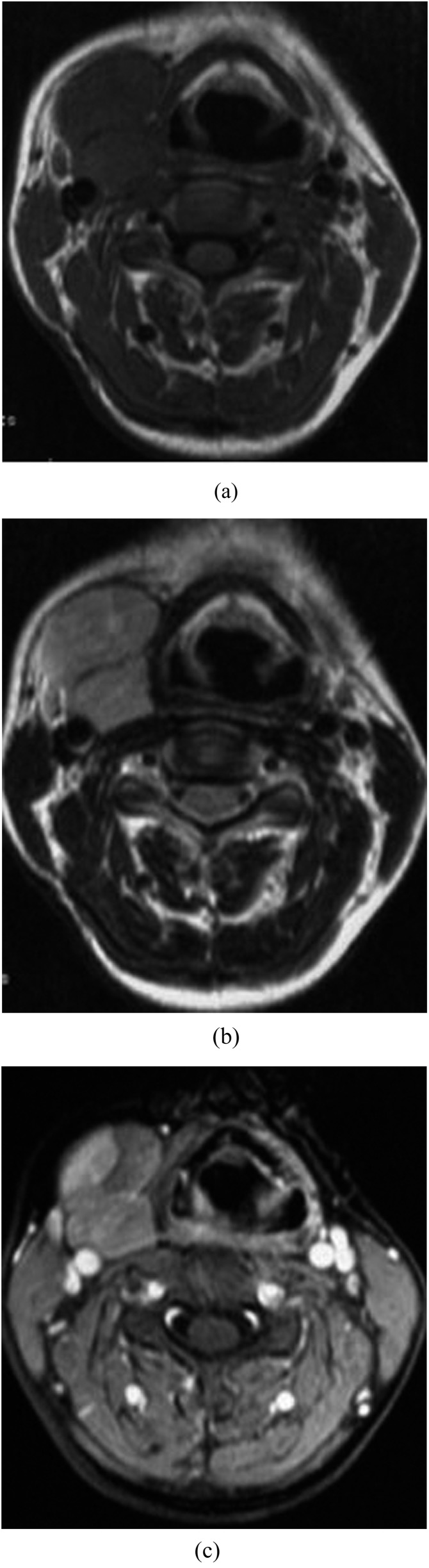
Figure 2.
Synovial sarcoma in the submaxillary space of a 26-year-old female. (a) A homogeneous lobulated mass in the submaxillary space with marked margin exhibits isointense signal on T1 weighted MRI. (b) The mass shows hypersignal together with hyposignal intensity fibrous septum on axial T2 weighted MR image. (c) The mass reveals moderate enhancement with focal notable enhancement.
Figure 4.
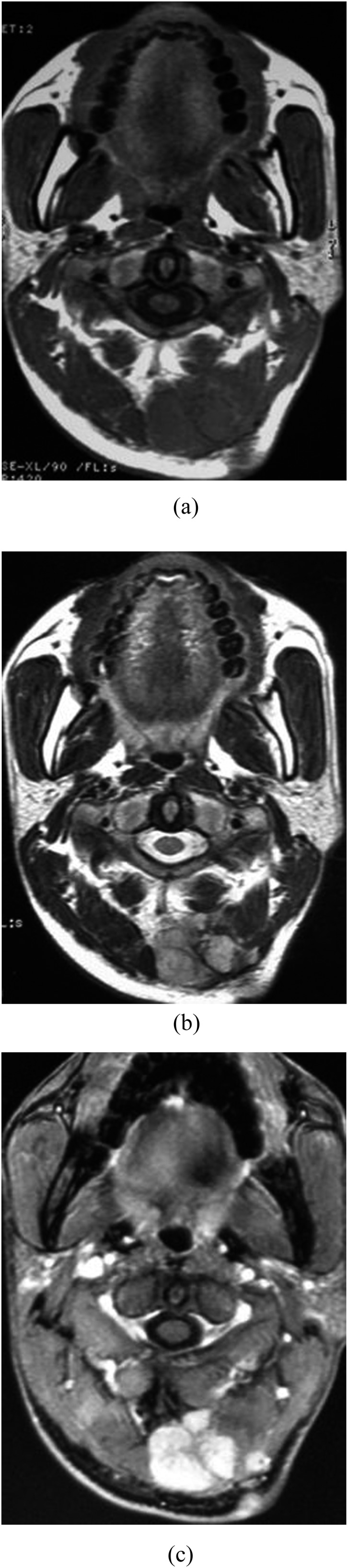
Synovial sarcoma in the retrovertebral space of a 24-year-old male. (a) A lobulated mass with fibrous septum shows isointense signal on T1 weighted MRI. (b) The lobulated mass shows hyper on T2 weighted MRI with hypo fibrous septum. (c) The lobulated mass shows notable enhanced parenchyma and mild enhanced fibrous septum.
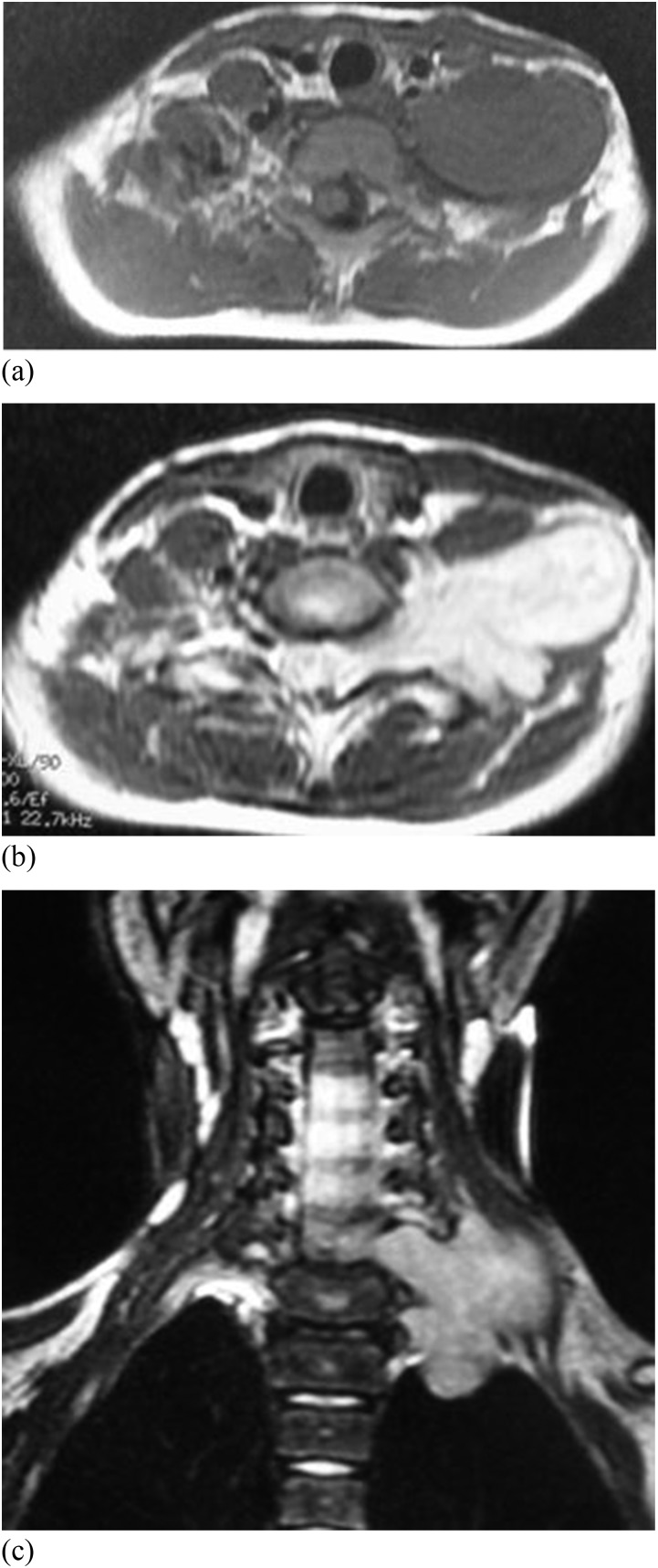
Figure 3.
Synovial sarcoma in the right temporomandibular space of a 38-year-old male. (a) Axial T1 weighted MR image shows isointense signal with focal hyper owing to haemorrhage. (b) Axial T2 weighted MR image shows the moderate hyperintense signal of tumour parenchyma and hyperintense signal owing to cystic degeneration. (c) Coronal T2 weighted MRI shows the moderate hyperintense signal of tumour parenchyma and cystic degeneration. (d) Coronal enhanced T1 weighted MR image shows remarkable enhancement of the mass parenchyma and unenhanced cystic degeneration.
None of the masses showed evidence of bone invasion, or local lymph node and lung metastases.
Pathological features
Four lesions received surgical ablation. They were all monophasic fibrous-type SS, which consisted of only spindle cells (Figure 6a). The IHC results of four patients were collected (Table 2). Epithelial membrane antigens (EMAs) were all positive. The positive rate of cytokeratin (CK) was 50% (2/4). The positive rate of part pan-CK antibody (AE1/3) and vimentin (Vim) was both 75% (3/4).
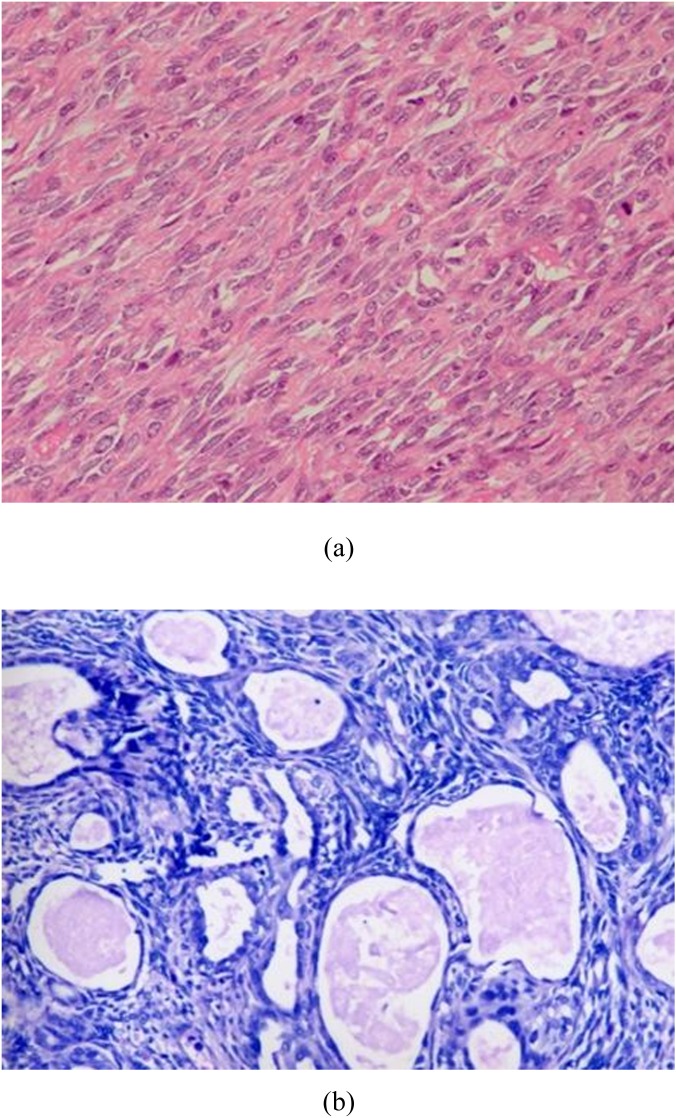
Figure 6.
The light microscopic features of monophasic fibrous-type and biphasic-type synovial sarcoma (SS). (a) Monophasic fibrous SS typically appears with fascicles and sheets of uniform small oval neoplastic cells. (b) Biphasic SS has (blue) spindle cell mesenchymal components and (pinker) glandular elements. For colour images see online.
Table 2.
Immunohistochemistry (IHC) result of four monophase synovial sarcomas
| Number | IHC+/− |
|---|---|
| Case 1 | AE1/3+(part), CAM 5.2+(part), EMA+, BCL-2+, calponin+, CD34− |
| Case 2 | Vim+, AE1/3+(part), CK+, EMA+, BCL-2+/−, CD99−/+, calponin−/+, S-100−, CD34− |
| Case 3 | Vim+, CK+(part), EMA+(part), CD99+, BCL-2−, SMA−, S100−, desmin− |
| Case 4 | Vim+, BCL-2+, AE1/3+(part), EMA+(part), S-100−, CD34−, SMA− |
+, positive; −, negative; AE1/3, pan-cytokeratin antibody; BCL-2, BCL-2 protein; CK, cytokeratin; EMA, epithelial membrane antigen; S-100, S-100 protein; SMA, smooth muscle actin; Vim, vimentin.
The only lesion located in the pharynx nasalis was confirmed by biopsy under endoscopy; first treated with radiotherapy and surgically removed eventually. It was a biphasic type of SS consisting of both spindle and epithelial cells (forming glandular structures) (Figure 6b).
DISCUSSION
SS accounts for 5–10% of all soft-tissue sarcomas and is the fourth most common type of sarcoma, following malignant fibrous histiocytoma, liposarcoma and rhabdomyosarcoma.3,5–7 SS may appear in any part of the body at any age. About 80–95% of SSs are located in the extremities, especially in the lower limbs around the knee joint.5,6,8
SS of the head and neck is unusual. The most common location in the head and neck is the hypopharynx.5,9,10 In our study, the masses were located in the paravertebral space near vertebral minor joint (zygapophysial joints), the submaxillary space, the retrovertebral space adjacent the atlanto-occipital joint, the nasopharynx and the temporomandibular space around the temporomandibular joint, respectively. These locations were rare. The biphasic-type SS mimicking nasopharyngeal carcinoma was the fourth ever documented nasopharyngeal SS.11 The SS in the paravertebral space with intraspinal involvement was diagnosed as neurogenic tumour pre-operationally. The SS in the submaxillary space, with well-defined margin and notable enhancement, was thought to be pleomorphic adenoma originally. The SSs around temporomandibular joint and in the retroveterbral space were also misdiagnosed. These tumours mimic benign ones, which made the diagnosis challenging.
The symptoms of SS may vary with the location of the mass. Two cases revealed local slow-growing painless masses (n = 2); one case showed weakness of the upper limbs (n = 1); one case had difficulty of opening the mouth (n = 1); and one case complained about epistaxis (n = 1). SS is the most prevalent in adolescents and young adults. The ages of the five patients ranged from 12 to 38 years, with an average of 26 years. In other studies, males appeared to be slightly more susceptible than females.3,6,9 This was different from our findings, and it may be owing to the small sample size of our study.
MRI is the choice of modality for soft-tissue tumour, owing to its soft-tissue specificity and ability to define a mass in multiple planes.5,6 A heterogeneous mass with “triple sign” (hypo-, iso- and hyperintense signal on T2WI) that represents the existence of different tissues, such as fibrous tissue, haemorrhage, cystic degeneration and fluid–fluid level is characteristic for SS.3,5,12–14 The radiological features of SS in the head and neck are controversial.9,15 In this group, these lesions were mainly solid and homogeneous and appeared as isointense signals similar to that of the skeletal muscle on T1WI and as hyperintense signal on T2WI with remarkable enhancement. Fibrous septum emerged in two lesions with hypointense signal on both T1 and T2 MRI with mild enhancement. Haemorrhage with high signal intensity on T1WI/T2WI was revealed in one mass; cystic degeneration was observed in one mass as hypointense signal on T1WI and as hypersignal intensity on T2WI with no enhancement. CT is helpful in detecting subtle calcifications, which is a common feature of SS.5,6 In our study, only one case had CT scans, and no apparent calcifications were found. All the lesions were well defined and mimicked benign masses, without adjacent bone, lung or lymph node involvement, except for one mass that invaded into the spinal canal via the intervertebral foramen and also involved the apex of the left lung. Another feature that may facilitate the diagnosis is that three of the masses were located adjacent to the minor joint in the head and neck.
Histopathological examination is of great help for the final diagnosis. Four of the five patients were monophasic fibrous-type SS and one was the biphasic-type SS in our study. Although there are four pathological subtypes of SS, the biphasic type consisting of distinct epithelial and spindle-cell elements and the monophasic fibrous type consisting of only spindle cells are more common.2,6 EMAs were positive in all four patients. The positive rate of CK was 50% (2/4). The positive rate of part AE1/3 and Vim were both 75% (3/4). The positive staining of EMA, Vim, CK and AE1/3 contribute to the diagnosis of SS.5,12 Most cases of SS can be diagnosed by haematoxylin–eosin staining and additional IHC staining of tissue specimens. The gold standard is typically accepted as a reciprocal translocation t(x;18)(p11;q11).3,13,16
Several types of tumours in the head and neck region should be differentiated from SS. Squamous cell carcinoma, the most common neoplasm of the head and neck region, is an infiltrative tumour with irregular margins, sometimes indistinguishable from the adjacent soft tissues, and frequent metastasis to the cervical lymph node. Neurogenic tumours (schwannoma and neurofibroma) and tumours of submandibular gland (pleomorphic adenoma) should also be differentiated.
Surgical ablation is the modality of choice for treatment.5,10,14,17 Surgical excision with negative margins is recommended. SS of head and neck has potential of recurrence.10,14,17 Post-surgery radiotherapy is needed to reduce local recurrence. Four lesions received surgical ablation; the lesion located in the nasopharynx was treated with radiotherapy at first and excised eventually.
CONCLUSIONS
We documented the fourth nasopharyngeal SS, which is extremely rare. SS with typical radiological manifestation is easy to diagnose. But the diagnosis for SS with uncommon findings remains challenging. A combination of several imaging features should provoke the diagnosis of SS: adolescence or young adult patients with slow-growing well-defined mass in head and neck, mainly solid and homogeneous, frequently adjacent to the minor joint; isointense signal similar to that of the skeletal muscle on T1WI, hyperintense signal on T2WI with remarkable enhancement; bearing fibrous septum, haemorrhage and cystic degeneration. The light microscopic characteristics and positive staining of Vim, AE1/3, EMA and CK could lead to a definite diagnosis.
FUNDING
This research was supported in part by grants from the Science and Technology Council of Shanghai (grant no. 15ZR1408000 and grant no. 18.no. 12140901302).
Contributor Information
P A Hu, Email: hpa222@126.com.
Z R Zhou, Email: zhouzr_16@126.com.
REFERENCES
- 1.Jernstrom P. Synovial sarcoma of the pharynx; report of a case. Am J Clin Pathol 1954; 24: 957–61. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology 2006; 48: 3–12. doi: 10.1111/j.1365-2559.2005.02284.x [DOI] [PubMed] [Google Scholar]
- 3.Nishiguchi T, Mochizuki K, Nakayama T, Inoue Y, Ohata K, Wakasa K. A case of synovial sarcoma in the perivertebral space of the neck: clinical presentation, radiological findings and histopathological description. Br J Radiol 2008; 81: e72–4. doi: 10.1259/bjr/86130609 [DOI] [PubMed] [Google Scholar]
- 4.Jang JW, Lee JK, Seo BR, Kim SH. Synovial sarcoma of the posterior neck: a case report and review of literature. J Korean Neurosurg Soc 2010; 47: 306–9. doi: 10.3340/jkns.2010.47.4.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Sullivan PJ, Harris AC, Munk PL. Radiological features of synovial cell sarcoma. Br J Radiol 2008; 81: 346–56. doi: 10.1259/bjr/28335824 [DOI] [PubMed] [Google Scholar]
- 6.Ulusan S, Kizilkilic O, Yildirim T, Hurcan C, Bal N, Nursal TZ. Radiological findings of primary retroperitoneal synovial sarcoma. Br J Radiol 2005; 78: 166–9. doi: 10.1259/bjr/67990800 [DOI] [PubMed] [Google Scholar]
- 7.Waldt S, Rechl H, Rummeny EJ, Woertler K. Imaging of benign and malignant soft tissue masses of the foot. Eur Radiol 2003; 13: 1125–36. doi: 10.1007/s00330-002-1604-y [DOI] [PubMed] [Google Scholar]
- 8.Nakajima H, Matsushita K, Shimizu H, Isomi T, Nakano Y, Saito M, et al. Synovial sarcoma of the hand. Skeletal Radiol 1997; 26: 674–6. doi: 10.1007/s002560050310 [DOI] [PubMed] [Google Scholar]
- 9.Hirsch RJ, Yousem DM, Loevner LA, Montone KT, Chalian AA, Hayden RE, et al. Synovial sarcomas of the head and neck: MR findings. AJR Am J Roentgenol 1997; 169: 1185–8. doi: 10.2214/ajr.169.4.9308488 [DOI] [PubMed] [Google Scholar]
- 10.Amble FR, Olsen KD, Nascimento AG, Foote RL. Head and neck synovial cell sarcoma. Otolaryngol Head Neck Surg 1992; 107: 631–7. [DOI] [PubMed] [Google Scholar]
- 11.Nakahira M, Sugasawa M, Morita K. Monophasic synovial sarcoma of the nasopharynx. Auris Nasus Larynx 2013; 40: 413–16. doi: 10.1016/j.anl.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 12.Jones BC, Sundaram M, Kransdorf MJ. Synovial sarcoma: MR imaging findings in 34 patients. AJR Am J Roentgenol 1993; 161: 827–30. doi: 10.2214/ajr.161.4.8396848 [DOI] [PubMed] [Google Scholar]
- 13.Bakri A, Shinagare AB, Krajewski KM, Howard SA, Jagannathan JP, Hornick JL, et al. Synovial sarcoma: imaging features of common and uncommon primary sites, metastatic patterns, and treatment response. AJR Am J Roentgenol 2012; 199: W208–15. doi: 10.2214/AJR.11.8039 [DOI] [PubMed] [Google Scholar]
- 14.Bixby SD, Hettmer S, Taylor GA, Voss SD. Synovial sarcoma in children: imaging features and common benign mimics. AJR Am J Roentgenol 2010; 195: 1026–32. doi: 10.2214/AJR.10.4348 [DOI] [PubMed] [Google Scholar]
- 15.Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. AJNR Am J Neuroradiol 2001; 22: 851–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Jain S, Xu R, Prieto VG, Lee P. Molecular classification of soft tissue sarcomas and its clinical applications. Int J Clin Exp Pathol 2010; 3: 416–28. [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Yang L, Li Q, Chen M, Zhang H. Synovial sarcoma of the head and neck—a retrospective study of 39 cases. [In Chinese.] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013; 27: 1167–70. [PubMed] [Google Scholar]