Abstract
Nanoparticulate delivery systems represent an area of particular promise for nanoneuromedicines. They possess significant potential for desperately needed therapies designed to combat a range of disorders associated with aging. As such, the field was selected as the focus for the 2014 meeting of the American Society for Nanomedicine. Regenerative, protective, immune modulatory, anti-microbial and anti-inflammatory products, or imaging agents are readily encapsulated in or conjugated to nanoparticles and as such facilitate the delivery of drug payloads to specific action sites across the blood-brain barrier. Diagnostic imaging serves to precisely monitor disease onset and progression while neural stem cell replacement can regenerate damaged tissue through control of stem cell fates. These, taken together, can improve disease burden and limit systemic toxicities. Such enabling technologies serve to protect the nervous system against a broad range of degenerative, traumatic, metabolic, infectious and immune disorders.
Keywords: Nanoneuromedicine, Nanotechnology, Nanoformulation, Drug development, Targeting
Graphical abstract
Nanoneuromedicine represents a new class of nanotechnology-enabled approaches for targeted delivery 16 of therapeutics and for control of cellular process
Introduction
Nanotechnology and nanomedicine approaches involving therapeutics and diagnostics have had a huge impact on medicine notably for cancer, developmental, infectious and immune disorders. However, until very recently, nanomedicine approaches have not been as deeply developed for the neurosciences. Nanoneuromedicines possess significant potential as opportunities abound in the development of desperately needed therapies and diagnostics to combat degenerative, inflammatory, infectious and genetic disorders associated with aging. This growing field was selected as the focus for the 2014 meeting of the American Society for Nanomedicine.1
A fundamental hurdle in developing effective therapies for nervous system disorders resides in an inherent inability of nerve cells to regenerate and/or even repair modest damage incurred to the brain and spinal cord.2 Nervous system injury follows a variety of insults such as stroke, trauma, developmental disorders, aging, malignancy, chemical exposures or microbial infections.3-7 Typical treatment options utilized, or in development, include therapeutic symptomatic management, stem cell implantation, neural tissue grafts or guidance strategies.8-12 Another significant challenge associated with improving nervous system function includes transport of therapeutics across the blood-brain barrier (BBB). Typically, the therapies need to be delivered to the site of the nervous system malfunction and be available long-term. This could be overcome by surgical delivery of therapies to the affected brain and spinal cord sites. Alternative approaches are site-directed drug delivery. However, in contrast to other regions of the body, the nervous system poses unique challenges to site-specific drug delivery as the BBB moderates entry of substances into the brain.13
Nanotechnology approaches offer several opportunities to overcome these challenges, including the ability to circulate drug for extended times and to permit functionalization with targeting moieties to promote transport across cell membranes.6,14 This could facilitate the use of multifunctional therapeutic, imaging and diagnostic devices, called theranostics.15
Drug targeting to specific locations is needed for enabling delivery across the BBB and for controlling the fate and behavior of the stem cells in stem cell-based therapies. This review surveys recent developments in delivery systems for nanomedicines that cross the BBB and those that affect stem cell repair or regeneration (Figure 1). These nanotechnology approaches serve as enabling technologies in the emerging field of nanoneuromedicine related to applications in diagnostics, imaging and therapeutics of relevance to the nervous system.
Figure 1.
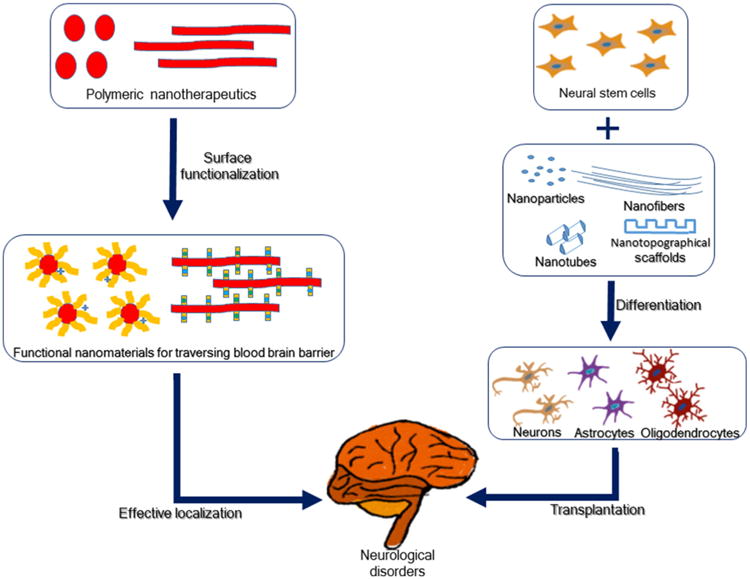
Nanotechnology approaches for targeted delivery of therapeutics and for control of stem cell behavior. These are outlined in box designations in their utilities to address neurological disorders.
Advances in polymer chemistry and nanoparticle delivery for central nervous system (CNS) targeting
In many cases, nervous system targeted therapies include antioxidants, anti-inflammatory agents, immunomodulatory compounds, growth factors, genes, siRNA and anti-microbials. The rational design of particles for central nervous system (CNS) drug delivery should take into consideration both drug-polymer compatibility and BBB transport. Development of a CNS targeting strategy is dictated by the desired surface properties of the particulate drug carrier. In this context, particle chemistry, particle surface modification and functionalization, and drug targeting strategies are discussed.
Particle chemistry
Nanoparticles can provide targeted delivery to specific areas of the nervous system by choice of appropriate sizes and chemistries. Several classes of biodegradable polymers have been studied for CNS delivery and include polyalkyl cyanoacrylates, polyesters, polyanhydrides, and polyethers. These polymers demonstrate tunable erosion profiles, easily modified surface chemistry, and sustained payload release profiles.16,17 The chemistries are summarized in Table 1.
Table 1.
Summary of polymeric nanoparticle use for enhanced CNS drug delivery.
| Approach | Nanoparticle properties | Targeting | In vivo efficacy | Ref | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| Core polymer |
Linkage | Surface coating |
Size (nm) |
PDI/ GSD |
Z-Pot. (mV) |
Payload | Encap. % |
Ligand | Amt. | Admin Route |
Dosage (mg/kg) |
Response | ||
| Receptor | PBCA | Acrylate | PS80 | 230 | NA | NA | Dalargin | 30 (S) | NA | NA | i.v. | 7.5 | 42.5 (28.9) % MPE | 18 |
| Receptor | PBCA | Acrylate | PS20 | 230 | 0.1 | NA | Dalargin | 30 (S) | NA | NA | i.v. | 10 | 7 | 20 |
| PS40 | 85.2 (20.7) % MPE | 7.4 (25.9) % MPE | ||||||||||||
| PS80 | 97.7 (4.6) % MPE | |||||||||||||
| C40 | 17.9 (16) % MPE | |||||||||||||
| Receptor | PBCA | Acrylate | PS80 | 270 | NA | NA | Doxorubicin | 80 (S) | NA | NA | i.v. | 5 | 6 μg/g | 21 |
| Receptor | PBCA | Acrylate | PS80 | 270 | 1.07 | NA | Doxorubicin | 70 (S) | NA | NA | i.v. | 3 × 2.5 | 43 % IST | 22 |
| Receptor | PBCA | Acrylate | PS80 | 290 | 0.08 | NA | Loperamide | 47 (S) | NA | NA | i.v. | 3.6 | 53.9 (34.2) % MPE | 23 |
| Receptor | PLGA/PVA | Ester | PS80 | 239.9 | 0.187 | 8.2 | Doxorubicin | 75 | NA | NA | i.v. | 3 × 1.5 | 40 % LTS | 27 |
| P188 | 242.4 | 0.211 | 6.0 | i.v. | 40% LTS | |||||||||
| PLGA/HSA | P188 | 408.6 | 0.289 | 8.1 | 97 | 25% LTS | ||||||||
| PLGA/PVA | PS80 | 166.9 | 0.266 | −25.0 | Loperamide | 77 | 7 | 80% MPE | ||||||
| P188 | 168.5 | 0.346 | −17.9 | 80% MPE | ||||||||||
| PLGA/HSA | PS80 | 292.4 | 0.092 | −18.9 | 82 | 40% MPE | ||||||||
| P188 | 287.7 | .077 | −17.5 | 50% MPE | ||||||||||
| Receptor | PLGA/HSA | Ester | P188 | 468 (19) | .404 (.158) | −11.2 | Doxorubicin | 88.5 | Lecithin | 7% | i.v. | 3 × 2.5 | -12.1 (24.1) μg/mm2 | 31 |
| Receptor | Chitosan-PEG | Ether | NA | 637 (2) | NA | 18 (4) | Z-DEVD-FMK | 23 (1) | Transferrin Receptor Mab | NA | i.v. | 1 | PEC | 62 |
| Receptor | PBCA | Acrylate | NA | 300 | 0.177 | NA | Dalargin | (S) | Apo B | 12.5 μg/mL (S) | i.v. | 7.5 | 15.17 (14.11) % MPES | 107 |
| Apo E | 26.08 (21.43) % MPE | |||||||||||||
| PS80 | Apo B | 64.68 (25.61) % MPE | ||||||||||||
| Apo E | 52.09 (11.22) % MPE | |||||||||||||
| NA | Loperamide | (S) | Apo E | 3.6 | 52.8 (35.5) % MPEP | |||||||||
| PS80 | NA | 96.7 (12.1) % MPE | ||||||||||||
| Apo E | 96.7 (12.1) % MPE | |||||||||||||
| Receptor | PEG-PLGA (50:50) | Ester-Ether | NA | 120 | NA | −14 | Urocortin | NA | Lactoferrin | 42/particle | i.v. | 28 μg | 25% | 87 |
| Adsorption | PLGA | Ester | P188 | 155 (26) | 0.13 (0.01) | −15.2 (5.6) | Loperamide | 15.1 (0.7) | g7 peptide | 39 umol/g | i.v. | 2.7 | 60% MPE | 108 |
| Receptor | PEG-PLGA | Ester-Ether | NA | 132 | NA | −21.42 | Novel active | 57.52 | NA | NA | i.v. | 4 | PPT | 29 |
| Adsorption | (25:75) | NA | 151 | −19.59 | peptide | 48.18 | TGN | 25% | 1 | PEC | ||||
| Receptor | PLA | Ester | PVA | 300 or 125TEM | 0.1 or 1.05 | −19.3 (0.5) | Ritonavir | 89.7 | NA | NA | i.v. (d10) | 45 | 10 μg/g | 30 |
| Adsorption | 157TEM | 0.14 or 1.06 | 2.4 (0.3) | TAT | 0.23 μg/mg | 80 μg/g | ||||||||
| Adsorption | P407-Chitosan | Ester-Ether | PEG | 148 (31) | .30 (.01) | 12.1 (0.8) | β-galactosidase | >90 | RVG29 | 1.8% | i.v. | 5 | ∼25% of dose | 63 |
| Cell | NA | NA | P407 | 383 | NA | −10.2 | Atazanavir | 100 (H) | NA | NA | s.c. | 2 × 250 | 10.6 ng/g | 105 |
| 365 | −24.6 | Folate | 40% | 33 ng/g | ||||||||||
| 471 | −21.5 | Ritonavir | NA | 4.1 ng/g | ||||||||||
| 454 | −18.3 | Folate | 40% | 34.5 ng/g | ||||||||||
Values inside parenthesis are composition for core polymer or standard deviation for numerical values.
NA – Not applicable or not analyzed in reference.
Size – Hydrodynamic diameter determined from DLS unless otherwise noted.
Encap % – percentage of drug encapsulated into core polymer. (S) – surface absorption of drug. (H) – core is homogenized drug.
i.v. – intravenous administration via tail vein.
s.c. – subcutaneous administration.
Dosage – mg drug/kg mouse unless otherwise noted. Multiple administrations are listed as number of administrations × dose of drug for each administration.
Response – Various methods utilized to evaluate response compared to either soluble drug or nanoparticle drug without targeting modification: concentration (μg/g, ng/g) given as mass of drug present per mass of brain tissue; PEC – performance equivalent to control; PPT – positive performance to test; MPE – maximal possible effect; LTS – long-term survival; IST – increased survival time.
Poly(alkyl cyanoacrylates)
Dalargin, a hexapeptide, adsorbed to the surface of poly(butyl cyanoacrylate) (PBCA) nanoparticles and coated with the surfactant polysorbate 80, provided the first successful delivery of a peptide administered by intravenous injection to the CNS.18 In this and related work, polysorbate 80 coating of the nanoparticle proved essential for BBB penetration. PBCA is readily biodegradable with no toxic metabolites and is rapidly cleared.19 The rate of degradation can be modified by substitution of the alkyl group, but these substitutions also affect metabolite toxicity. This is the most well-established polymeric nanoparticle delivery system for crossing the BBB, and has been loaded with compounds that include the hexapeptide dalargin, 18,20 doxorubicin, 21,22 loperamide, 23 and tubocurarine.24 Therapeutics predominantly were adsorbed onto the PBCA nanoparticle surface after polymerization. This decouples the release of the drug from PBCA degradation, often resulting in poor controlled release.
Polyesters
The biomedical applications of polyesters have been known for more than 40 years. Degradable polyesters were investigated for CNS delivery and include poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA).25 Polyesters are commercially available and approved for human use by the U.S. Food and Drug Administration (FDA, Silver Spring, MD) making them promising candidates for use as biodegradable platforms. One of their most important properties is their low cytotoxicity attributable to their rapid degradation into metabolites that are quickly processed by cells.26 Additionally, the preparation of polymers into nanoparticles is such that the therapeutic agent can be incorporated into the polymer matrix, coupling their release to polymer degradation kinetics.25,26 Surface modifications can be performed either by altering the polymer prior to particle formation or by conjugation to the surface post-particle formation. There are advantages and disadvantages to both methods of surface modifications. CNS delivery of drugs is enhanced by polyester core nanoparticles including, but not limited to, loperamide,27,28 active peptides,29 ritonavir,30 and doxorubicin.27,31
Even through the use of polyesters for drug delivery, shortcomings remain for their general use. Notably, all polyesters undergo bulk erosion due to the stability of ester bonds,32,33 which often result in rapid, or burst, release of drug payloads.28,31,34-36 Degradation, notably, depends on the backbone chemistry. Polyglycerol adipate is more hydrophilic because of the single carbon chain compared to PLA, which has a two-carbon chain backbone and shows much slower degradation. The lactic acid component of PLGA can easily be varied between 50 and 100% and the release profiles of encapsulated payloads extended.36,37 The molecular weight of the polymer can be varied to marginally control the release of payload.35,36,38 Coating the PLGA surface with hydrophobic materials, like gelatin or chitosan34, can decrease the initial drug burst and extend the release period. The rapid degradation of polyester products occurs in microenvironments with a low pH (1.5 – 3.6),39,40 which can be problematic when the therapeutic payload is denatured in parallel.
Polyanhydrides
Polyanhydrides possess good biocompatibility and drug delivery potential. This has fostered significant research with these materials.41 Degradable polyanhydrides were developed for CNS delivery and include sebacic acid and 1,3 bis(p-carbox-yphenoxy)propane. Polyanhydrides specifically developed for CNS delivery included implantable wafer systems for the treatment of Alzheimer's disease42,43 and for brain cancer.44-46 These polyanhydride implants are degraded into biocompatible metabolites and are readily eliminated.47 The design and commercialization of the Gliadel® wafer, a FDA approved implantable device for the controlled release of carmustine, is an example of a successful polyanhydride implant48 and is inserted following surgical removal of brain tumors.
The preparation of polyanhydride nanoparticles allows for the incorporation of drugs within a polymer matrix, which enables the release of the payload with the polymer degradation. With most backbone chemistries (aliphatic or aromatic hydrocarbons), polyanhydride-based devices are surface degrading.32,33 By varying the degree of hydrophobicity based on backbone chemistry, polyanhydride devices can rapidly degrade (days), or very slowly degrade (over one year), and as such control drug release.49-51 The incorporation of moieties (e.g., ethylene glycol) within the polymer backbone shifts the degradation towards a combination of bulk and surface erosion.17 Polyanhydride particles are also affected by surface modification of the terminal carboxylic acid groups.52,53 The monomers released from polyanhydride degradation are not as acidic (4.2 – 6.5) as those seen during polyester degradation.39,40 Surface erosion, along with a wider range of pH microenvironments make polyanhydrides promising carrier materials. However, polyan-hydrides are highly susceptible to hydrolytic degeneration with the half-lives of the anhydrides six orders of magnitude greater than polyesters.33 Partially due to this hydrolytic susceptibility, they are not as translatable as the other polymer chemistries.
Polyethers
Synthetic and naturally inspired polyethers have been used in polymeric drug delivery for over 30 years.54-56 Poly(ethylene glycol) (PEG) and poly(propylene glycol) (PPG) have been used as triblock pluronics ([PEG]n-[PPG]m-[PEG]n) together with other polymers. Those naturally derived polymers such as chitosan, a cationic polysaccharide, are promising drug delivery vehicles.57,58 Polyethers are not very susceptible to hydrolytic degradation since their ether bond is very stable in water. Instead, polyethers can be degraded either by enzymes, through oxidation or by dissociation prior to excretion. While there are specific enzymes for chitosan, degradation of the synthetically derived polyethers has only been reported in bacterial cultures.59,60 Without a biodegradation mechanism, polyether particles synthesized from synthetically-derived polyethers are inert.61 For CNS delivery, polyether particle cores can either incorporate chitosan62,63 or be incorporated into the backbone of other polymers to facilitate desired amphiphilicity in polyanhydrides.64,65
Particle surface modification and functionalization
While the route of administration can affect the bioavailability of nanotherapeutics, intravenous injection remains the preferred delivery method for evaluating CNS nanotherapeutic efficacy. When administered intravenously, nanotherapeutics first interact with the plasma in the circulation. Particle size, surface chemistry, hydrophobicity and charge are all known to greatly influence the absorption of proteins, cellular interactions and duration of circulation.66-68 The presence of a PEG corona on the particle surface also alters the profile of absorbed serum proteins on the surface when administered intravenously.69 Additionally, these surface coatings have been observed to reduce clearance through the reticuloendothelial system (RES).68,70 Only the polysorbates result in biological effects after intravenous injection, with polysorbate 80 having the greatest efficiency.20 This has also been shown to occur in other drug-PBCA nanoparticles containing doxorubicin.21 Coating PBCA with polysorbate 80 can facilitate delivery of particle therapeutics across the BBB, although the exact mechanism of enhancement is unclear.71,72 In a comparison of surfactants, multiple polysorbates (20,40,60,80), multiple poloxamers (184, 188, 388, 407, 908), Brij 35 and Cremophors (EZ, RH 40) were coated onto PBCA nanoparticles with dalargin adsorbed to the surface. The current consensus is that polysorbate 80 on the surface affects the type of serum proteins which are adsorbed, influencing transport across the BBB.73 An alternative route of administration is intranasal administration that has received considerable attention in recent years.66-68,74,75 Properties of the core polymers, including additional modification of the particle surface either by polyethers (specifically PEG), stabilizers or surfactants is commonly used to enhance drug delivery to the CNS. Moreover, the choice of surface functionalization has significant implications on the overall effectiveness of delivery across the BBB.76
Polyethers are used as surface modifiers to alter the surface property of the core nanoparticle for CNS therapies. The surface attachment of PEG is generally accomplished through conjugation to the core polymer either pre- or post-particle synthesis, essentially forming a block copolymer. For example, the tri-block copolymer pluronic can be used to coat the surface of particles through adsorption as a stabilizer in emulsion particle synthesis. Drug devices and nanoparticles coated with PEG possess a steric stabilization effect in which the hydrophilic PEG opposes interactions with the host, especially phagocytosis and cellular adhesion.20,21,68-73,77-79 Surfactants, including pluronic, poly(vinyl alcohol) (PVA) and to a lesser extent human serum albumin (HSA), are used as stabilizers in many nanoparticle formation methods. While both PVA and HSA are biocompatible, PVA is not biodegradable.80 The use of these stabilizers controls the size of the particles synthesized, reduce the polydispersity of the synthesized particle size, and enhance drug encapsulation efficiency. However, the inclusion of these surfactants can alter the surface properties of the nanoparticle core, which can be more important in influencing penetration of the BBB.76 A summary of particle surface modifications and their impact on BBB penetration is shown in Table 1.
CNS nanoparticle drug targeting strategies
While particle core chemistry and surface modification can control the release of the payload and reduce RES clearance, neither directly addresses the mechanism by which they cross the BBB. One strategy to move nanoparticles across the BBB is to initiate transcytosis of the brain capillary endothelial cells (BCEC). This is accomplished by either binding a ligand for a surface expressed receptor on the circulation side of the BCEC (i.e., receptor-mediated) or by adsorbing the particle to the BCEC membrane, inducing endocytosis (i.e., adsorptive mediated). A second strategy to transverse the BBB is utilization of innate immune cells, like monocytes and macrophages that phagocytose nanoparticles with drug payload(s) and carry the drug within the cells across the BCEC (i.e., cell-mediated). A schematic of these strategies to transverse the BBB is depicted in Figure 2. While this section focuses on the use of these technologies to improve polymeric nanoparticle delivery of therapeutics across the BBB, these methods have also been applied to liposomes, solid lipid nanoparticles and inorganic nanoparticles. Recent reviews have discussed the utility of these additional delivery systems.25,81
Figure 2.
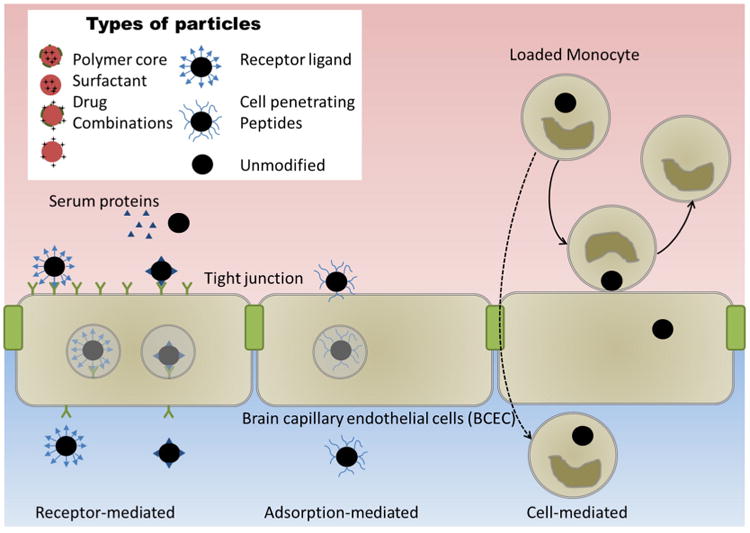
Strategies for nanoparticles to traverse the blood-brain barrier.
Endothelial transcytosis
Receptor-mediated
Receptors on the BCEC can be targets to improve nanoparticle uptake through receptor-mediated endothelial transcytosis and include low density lipoprotein receptor,69,82,83 transferrins,84-87 leptins,88 epidermal growth factor,89 diphtheria toxin,90 and insulin.91,92 Use of those cellular receptors and improved BCEC transport is attained by surface modification of nanoparticles with endogenous ligands, peptides derived from the endogenous ligands and antibodies against the receptors. A summary of particle surface modifications for receptor-mediated endothelial transcytosis and their impact on BBB penetration is provided in Table 1.
Receptor-mediated endothelial transcytosis engages proteins differently. In regards to brain endothelial cells, apolipoproteins adsorbed to the surfaces of polyhexadecylcyanoacrylate (PHDCA) nanoparticles are biologically distinct from those adsorbed onto nanoparticles of PEG-PHDCA copolymers.69 Apolipoprotein E (ApoE) and ApoB-100 absorbed on the surface of the PEG-PHDCA nanoparticles results in particle penetration of BCEC; whereas, the same amount of opsonizing proteins on PHDCA nanoparticles results in clearance without BCEC penetration.69 Two limitations to receptor-mediated endothelial transcytosis are the quantity of receptors on the BCEC surface that can limit the amount of transport and the lack of specificity of expression of these receptors for BCEC, thus limiting specificity of brain delivery and particle-receptor mediated transcytosis.93
Adsorption-mediated
Cell-penetrating peptides increase the delivery of nanoparticles across the BCEC by adsorption-mediated transcytosis. Examples include the human immunodeficiency virus type 1 transactivator of transcription protein,30,94,95 poly-arginines,96 and Syn-B vectors.97,98 The herpes simplex virus type 1 peptide (gH625) has also been shown to increase the transport of polystyrene particles across the BCEC.99 A modified opioid peptide (g7) enhances BCEC penetration of nanoparticle-encased drugs by conformationally promoting macropinocytosis.100 After traversing the BCEC layer, additional modification of the cell surface with antibodies to cell specific markers was shown to enhance the specificity of g7-nanoparticle delivery.101 A summary of particle surface modifications for adsorption-mediated endothelial transcytosis and the in vivo impact on BBB penetration is outlined in Table 1.
Cell-mediated transcytosis
Cell-mediated transcytosis was first demonstrated utilizing serotonin-carrying liposomes in monocyte-macrophages.102 Such a transport method was also used in the delivery of antiretrovirals103 and catalase.104 Crystalline antiretroviral drug nanoparticles were rapidly taken up into human monocyte derived macrophages and subsequently transferred to BCEC.105 Experiments performed with catalase-loaded polymeric nanoparticles also showed enhanced brain delivery when the nanoparticles were pre-loaded into macrophages.106
For this method of delivery, the liposomes were not modified to be hydrophilic, neutrally charged or an ultra-small size, but rather to make the particles more amenable to phagocytosis. Similarly, folate surface modification of nanoformulated antiretroviral therapy particles to engage the folate receptor on macrophages resulted in transfer of more nanoparticles to BCECs in vitro and corresponded to pharmacodynamic benefits.105 A summary of the use of particles for cell-mediated transcytosis and their in vivo impact on BBB penetration are shown in Table 1.
Neural stem and neural progenitor cells
Neural stem cells and neural progenitor cells can play key roles in addressing neurodegenerative disorders. Studies demonstrating the importance of nanoscale materials and features that help to regulate neural stem cell (NSC) adhesion, proliferation and differentiation into specific neural lineages are discussed. Although details defining mechanisms regulating NSC behaviors with nanomaterials remain to be clearly elucidated, a number of significant advances have been achieved that can be used for targeting specific neural tissue sites for delivery as well as nanotechnological approaches to control stem cell differentiation and behavior. Applications of nanotechnologies to address neurodegenerative disorders and infections of the nervous system have been developed. In addition to delivery of bioactive molecules, the use of NSC and neural progenitor cells (NPC) synergistically with nanotechnological approaches offers a novel opportunity to address treatments for nervous system disorders and may serve as mechanism(s) for repairing deficits after injury. This requires the development of methods for controlling the development and differentiation of these cells in ways that are relevant for their use in cell transplants or within implants to be used in a variety of CNS or peripheral nervous system (PNS) targets.
In a developing embryo, NSC can differentiate into all of the specialized cell types of the CNS and PNS. Of particular interest is the potential for use of human NSC in regenerative medicine to treat a range of conditions including spinal cord injury, Parkinson's disease (PD), amyotrophic lateral sclerosis and blindness. Moreover, the ability to use NSC as “off-the-shelf” cellular targets to improve drug design and validation for screening is of intense interest to pharmaceutical companies. NSC are also being studied to improve our fundamental knowledge of developmental principles as well as to improve our understanding of CNS birth disorders.
NSCs are multipotential progenitors of neurons and glia that have been isolated from the CNS. Like NSC, NPC have the capacity to differentiate into specific types of cells though they are somewhat more specified in their differentiation capacities.109 NSC and NPC offer several advantages for CNS repair. NPCs can proliferate in culture and can survive following transplantation into the brain, spinal cord and eye, which is being used as a basis for therapeutic approaches. Here they integrate and stably express foreign genes, or help replace damaged or diseased cells.109 They can be clonally expanded, providing a renewable supply of transplantable material. They can also be engineered to express exogenous genes for neurotransmitters and neurotrophic factors that can help neuron survival. Thus, it is possible that NPC may also be capable of integrating into host neural circuitry and/or supply trophic factors to enable cell survival and recovery.
NPC have been isolated from various regions of the CNS, such as the cortex, hippocampus, subventricular zone, spinal cord ependyma and retina.110-114 Considerable effort has been devoted to elucidate the stem cell microenvironment, or “niche”, controlling cell fate.115 A number of studies demonstrated that differentiating NPC are regulated not only via intrinsic genetic control, but also in large part by direct cell-to-cell contact and cell-to-extracellular matrix interactions, topographical control as well as by soluble factors.116-122 Such interactions can involve a complex “cocktail” of these signaling proteins.123
An important strategy to regulate NPC is to manipulate the microenvironment. Micro- and nanotechnology approaches have considerable potential to mimic the microenvironment in which NPC integrate at the site of injury or neurodegeneration.124-127 Nanomaterials have unique biomimetic characteristics and can manipulate biological and mechanical properties of this microenvironment.127 This can have profound influences on neural stem cell differentiation and functional integration.124-126 Different nanomaterial preparations including nanoparticles, nanofibers, nanotubes and nanotopographical scaffolds can be fabricated and applied to address critical requirements for cell control in repair and can best affect the microenvironment of the CNS.
Nanoparticles are commonly used for stem cell imaging and tracking; intracellular drug/trophic factor/plasmid DNA carriers to control stem cell proliferation and differentiation; and as biosensors to monitor intracellular levels of relevant biomole-cules/enzymes.128-132 Nanofibers and nanotopographical scaffolds have been used to direct cell fate during differentiation because they can be designed to mimic the microenvironment. Nanotubes are mostly used in tissue engineering due to their mechanical, electrical and thermal properties. Each of these nanoengineered systems can have a broad range of applications for cell therapy to address a variety of neurodegenerative disorders.
Nanofibers
Nanofibers are an important tool in the field of tissue engineering as they can closely mimic the extracellular matrix architecture, and thus specifically be used to maximize cell-substrate interactions.133 Other benefits of nanofibers include drug delivery and tissue engineering scaffolds. In neural tissue engineering, they can act as a guidance cue for various cell types and sprouting axons. A number of studies have characterized the innate properties of biopolymeric nanofibers towards survival, proliferation and differentiation of NPC as an initial step before use for tissue engineering scaffold constructs. Common methods for fabricating nanofibers involve electro-spinning, phase separation and self-assembly.133,134 Electro-spinning (or electro-spraying) is a widely used method for creating nanofibers ranging from 50 nm to 1000 nm.133 NPC have been shown to selectively differentiate into various neuronal and glial cell types depending on the varying tunable properties of nanofibers. Graphene oxide has been shown to promote the growth and differentiation of adult stem cells and when coated in varying amounts on polycaprolactone nanofibers caused differing expression of neural markers in differentiated adult hippocampal NSC.135 Coating with high concentrations of graphene oxide resulted in differentiation to myelinating oligodendrocytes and also an increased expression of various molecules responsible for enhancing differentiation for oligodendrocytes during development. Retinoic acid induced differentiation for adult NSC and resulted in expression of neural differentiation markers when cells were cultured on nanofibers.136 Polysaccharide chitosan-derived nanofibers enhance both proliferation and differentiation of neurons and human NSC as compared to another polysaccharide, cellulose acetate.137 Ren et al fabricated nanofibers of varying diameters and alignment using polyether sulfone and optimized the differentiation of human pluripotent stem cell-derived neural crest stem cells towards a Schwann cell lineage.131 Electrical stimulation of NSC resulted in increased neurite outgrowth when cultured on electrospun nanofibers of poly-l-lactide (PLLA) blended with polyaniline (PANi) (PLLA/PANi nanofibers).138 Xu et al139 examined the efficacy of polyhydroxyalkanoate (PHA) nanofiber matrices by using three different types of PHA; poly(3-hydroxybutyrate) (PHB), copolymer of 3-hydroxybuty-rate and 4-hydroxybutyrate (P3HB4HB), and copolymer of 3-hydroxybutyrate and 3-hydroxyhexanoate (PHBHHx). All three PHAs in 2- or 3D matrices supported the growth and differentiation of NSC, but PHBHHx produced the most efficient NSC neuronal differentiation. Additionally, 3D had a greater advantage over 2D matrices in regards to NSC attachment and neurite formation. Immobilization of bioactive molecules on nanofibers has been tested for culturing NSC. Collagen was immobilized on nanofibers of copolymer of methyl methacrylate (MMA) and acrylic acid (AA) (PMMAAA) by an N-(3-dimethy-laminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS)IKV activation process.140 These collagen-immobilized nanofibers enhanced the attachment and viability of the cultured NSC. Coupling brain-derived neurotrophic factor (BDNF) to nanofibers is more effective in enhancing NSC proliferation and directing their differentiation toward neuronal and oligodendrocyte fates compared to soluble BDNF.141 Silva et al142 encapsulated murine NPC within a three-dimensional network of nanofibers formed by self-assembly of isoleucine-lysine-valine-alanine-valine -containing amphiphilic peptides. These nanofibers induced a selective differentiation of NSC towards neurons and reduced differentiation towards an astrocyte fate. In addition to NPC differentiation, nanofibers can differentiate embryonic stem cells to neurons.143-145
Nanoparticles
Nanoparticles, typically in the range of 1 to 100 nm are capable of acting as a whole unit in terms of size-related intensive properties. Properties of nanoparticles vary significantly compared to properties of bulk material due to the high surface area to volume ratio. In tissue engineering, nanoparticles are used for delivering therapeutic molecules such as drugs, antibiotics, growth factors, cytokines and other factors that can influence differentiation of stem cells.146-151 Magnetic nanoparticles have also been used for manipulating cellular function by using an applied external magnetic field.152,153 Both natural and synthetic polymers can be used for nanoparticle fabrication and encapsulating bioactive molecules. Magnetic nanoparticles made up of iron oxide and conjugated with CD133 were successfully used for isolation of NSC from ependymal cells of adult rats.154 All rats remained alive and healthy after the procedure and cells extracted were found to be capable of neuronal differentiation.
For cancer treatment that targets glioblastoma, a population of NSC displaying tumor-tropic migratory capabilities of NSC were loaded with pH sensitive doxorubicin-loaded mesoporous silica nanoparticles and used as self-destructive carriers.155 Nanoparticles have also been used for cell tracking. NSC loaded with iron oxide nanoparticles can be transplanted and subsequently tracked using noninvasive magnetic resonance imaging.128,131,156 Retinoic acid (RA) loaded nanoparticles using polyethylenimine (polycation) complexed with RA and dextran sulfate (polyanion) were used for controlling the differentiation of subventricular zone NSC by intracellular delivery of RA.157 A similar strategy was used for controlling mobilization and migration of human NSC by using hepatocyte growth factor (HGF) and leukemia inhibitory factor (LIF) loaded PLGA nanoparticles.158 Titanium dioxide (TiO2) coated with silicon dioxide (SiO2) selectively differentiate mouse NSC toward a neuronal phenotype. This can occur by altering nine different proteins involved in signaling, molecular chaperones, cytoskeleton and nucleoproteins.159
Nanotubes
Nanotubes are tubular structures with diameters of a nanometer scale (∼1-50 nm). They are considered to have a very large length to diameter ratio for any material. Carbon nanotubes (CNT) are most commonly used for tissue engineering160-162 because of their various electronic, thermal and mechanical properties. CNT are characterized into single-walled carbon nanotubes (SWNT) and multi-walled carbon nanotubes (MWNT) depending on the number of tubes (single/concentric). Arc discharge, laser ablation, chemical vapor deposition, liquid pyrolysis and ball milling are the methods generally used for CNT fabrication.160,163,164 A rope like structure with a diameter of 1 mm and length of 1.5 cm was created using CNTs fabricated by chemical vapor deposition.165 Electrical stimulation of NSC plated on these ropes caused them to differentiate into neurons at earlier stages compared to NSC growing on control conditions. Electrical stimulation promoted neuronal maturation and enhanced the speed of neurite outgrowth. In a different study, subventricular zone NSC were transplanted at the site of stroke in a rat model along with hydrophilic (HL) or hydrophobic (HP) CNT.166 HP-CNT reduced infarct cyst volume and increased expression of nestin, an NSC marker. Cell proliferation was increased with improved behavioral outcomes. Both HP-CNT and HL-CNT increased expression of microtubule-associated protein-2 (MAP-2) and reduced expression of glial fibrillary acidic protein (GFAP) suggesting that NSC differentiated towards a neuronal fate when transplanted along with these CNT. Parketal167 created CNT patternsbycreating a monolayer of CNT followed by selective adsorption of laminin on the CNT patterns. Human NSC grew selectively on these patterns and exhibited significantly different outgrowth behaviors. Mouse embryonic NSC from cortex were shown to differentiate into neurons, astrocytes and oligodendrocytes on glass coverslips coated with layer-by-layer assembled SWNT-polyelectrolyte multilayer thin films.168 Differentiation (neurite outgrowth and expression of neural markers) was comparable to NSC grown on glass coverslips coated with a poly-l-ornithine (PLO) substrate.
Nanotopographical scaffolds
Along with various biochemical cues, topographical cues have also been shown to alter growth, proliferation and differentiation of NSC.169,170 The morphology, alignment, focal adhesion assembly and differentiation of human NSC (towards neurons and astrocytes) was affected by fibronectin-coated polyurethane acrylate substrates with diverse nanoscale shapes (groove and pillar) and dimensions (ranging from 300 to 1500 nm groove width and pillar gap).171
Other promising nanomaterials
Various types of hybrid nanomaterials have been synthesized recently for imaging, therapeutic and biomedical applications. Hybrid nanomaterials are a combination of inorganic and organic nanomaterials, such that they not only exhibit the advantageous properties of the two materials involved but can also exhibit additional advantages of their own.172,173 These hybrid nanomaterials include technologies such as Nanoscale Metal– Organic Frameworks (NMOFs),174-176 functionalized nanotubes and nanogels. Lin and co-workers used a mesoporous silca-based nanoparticle system and cadmium sulfide nanocrystals as removable caps for controlled release of drugs and neurotransmitters.177 This particle system was found to be biocompatible and was used to investigate neurochemical interactions in astrocytes. Liposomes are closed bilayer phospholipid systems used for better entrapment and delivery of therapeutic drugs.178 Liposomes can also be used for virus free transfection to generate induced pluripotent cells,179 gene delivery to mesenchymal stem cells,180 targeting peripheral neurons and Schwann cells for enhanced uptake181 and targeting the CNS.182,183 Similarly, dendrimers are macromolecules of nanoscale dimensions with a central core, branched intermediate structure and then exterior functional groups. Combinations of various properties of these hierarchial components make dendrimers very promising candidates for drug delivery systems.184,185 Moreover, because of their antiamyloidogenic potential, they have also been used for the treatment of various neurological disorders such as Alzheimer's, PD and prion diseases.186,187
Altogether, nanomaterials and nanodevices have shown considerable promise in mimicking the nervous system's microenvironment, and thus can be used as effective tools for controlling NSC growth and differentiation. Functionalized nanoparticles using sugars and proteins by applying different bioconjugation techniques can resemble pathogens and target specific cell types. Cell targeting of nanoparticles during nervous system injury allows differentiation of stem cells into specific neurons or glia for controlling therapeutic cellular interventions. This approach may also reduce nonspecific deleterious bystander effects to the surrounding cells. Also, nanoscale patterning of proteins can be used for stimulating cells at the subcellular level that can affect cell migration, differentiation and proliferation. In the field of neural regeneration research, nanoscale patterning of a conduit with various neurotrophic factors can function as a guidance cue for regenerating axons. Aligned nanofibers have already been used for selective differentiation and alignment of NSC. Use of neurotrophic factor releasing nano- and micro-particles reflects a strategy for neuroprotection and neuroregeneration following spinal cord or other types of nerve injuries or neurodegeneration.
Although nanotechnology has produced positive results, a degree of caution is necessary, especially with respect to use of nanotechnology for NSC differentiation. Nanoparticles and nanotubes were found to be cytotoxic in some studies and decreased the proliferation of NSC. It has also been speculated that in some cases, immune cells may not be capable of recognizing nanoparticles all the time, and nanoparticles can pass unaided through the BBB itself. Other challenges associated with nanotechnology would be reducing the high cost associated with fabrication, scaling up production, improving the specificity for targeted cells and finally reducing the side effects that nanodevices may have on cells and other tissues.
Clinical applications
Nanotechnology is now considered as a potent tool to overcome various clinical challenges such as tissue engineering, drug delivery, imaging, diagnostics and therapeutics. While nanofibers are used for fabricating scaffolds to mimic a tissue microenvironment, and thus used for nerve, bone and other types of tissue engineering, nanoparticles have been used chiefly as drug delivery vehicles to control delivery of therapeutic agents at sites of injury and inflammation. Before using them for clinical applications, we need to understand the stability of these nanocarriers. Polymeric micelles have been known to improve the stability of hydrophobic drugs by encapsulating them inside or near to the hydrophobic core of the micelle. Hydrophilic chains on the outside help in enhancing in vivo compatibility and interaction of the micelles with tissues. Some important parameters that affect the stability of micellar carriers include lengths of the hydrophobic and hydrophilic blocks, chemical nature and molecular weight of hydrophilic blocks, physical state (amorphous or crystalline) of core forming polymer, pH sensitivity, interaction of micelle with serum proteins, thermodynamic stability above critical micelle concentration (CMC) and kinetic stability below CMC by having a stiff core.188,189 These nanodevices have also been used as tools to augment stem cell differentiation ex vivo. Furthermore, imaging the molecules of interest in vivo has become much simpler with the improvement in technologies associated with functionalizing nanoparticles. In addition, the use of nanoscale devices in clinical trials is on a constant rise since the approval of Doxil by the US FDA, the first FDA approved nanodrug.81,190-192 As of January 2012, of the 789 ongoing clinical trials, 25 involved nanodevices and 122 involved nanotherapeutics.191 By taking into account various peer reviewed publications, Weissig et al concluded that there are 43 nanopharmaceutical drugs that have been approved by the FDA (or equivalent foreign agencies).191 A high percentage of clinical trials (∼72% out of 6242 entries) involving nanodrugs were found to be related to cancer treatments.190 Nanoengineering as a manufacturing process and the necessity of nanomaterials for enhancing the therapeutic effect or enhancing functionality of existing drugs are the two main criteria for considering a drug as a nanopharamaceutical.191
Conclusions and outlook
As summarized above, nanotechnology and nanomedicine approaches serve as enabling technologies to overcome significant challenges associated with diagnosis, imaging and therapies to address malfunction of the nervous system. As described here, nanoscale systems with appropriate chemistries and functionalization can be extremely promising for safe, effective, targeted and site-specific, and sustained delivery of bioactive agents for imaging and to treat disorders of the nervous system. Nanomedicine offers new ways for therapeutics and imaging agents to traverse the BBB. A combination of delivery and stem cell-based therapies can significantly impact neuroregeneration. Future studies will continue to investigate strategies using nanotechnology to engineer scaffolds with various materials that can be used to regulate NSC fate decisions. Outcomes from these types of investigations are likely to provide important new information in designing and fabricating a 3D biomimetic neural stem cell niche. These enabling nanotechnologies can significantly impact diagnosis and therapies of nervous system disorders, which is outlined in detail in the accompanying review on clinical applications of nanoneuromedicine.
Acknowledgments
We would like to thank Robin Taylor for editorial support.
Funding: Funding for this work (Grant account no. W81XWH-11-1-0700) was provided by the US Army Medical Research and Material Command. University of Nebraska Medical Center research support includes individual donations from Dr. Carol Swarts and Frances and Louie Blumkin, the Vice Chancellor's office of the University of Nebraska Medical Center, ViiV Healthcare. SKM acknowledges the support of the Stanley Chair and BN acknowledges the Vlasta Klima Balloun Professorship. National Institutes of Health grants to researchers at Iowa State University and the University of Nebraska Medical Center: P01 MH64570, R01 MH104147, P01 DA028555, R01 NS036126, P01 NS031492, 2R01 NS034239, R01 AG043540, P01 NS43985, P30 MH062261, (HEG); and P20 GM103480 (TB) are gratefully acknowledged.
Abbreviations
- AA
acrylic acid
- Apo
apolipoprotein
- BBB
blood-brain barrier
- BCEC
brain capillary endothelial cells
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- CNT
carbon nanotubes
- FDA
U.S. Food and Drug Administration
- GFAP
glial fibrillary acidic protein
- HGF
hepatocyte growth factor
- HIV-1
human immunodeficiency virus type 1
- HL
hydrophilic
- HP
hydrophobic
- HAS
human serum albumin
- LIF
leukemia inhibitory factor
- MAP-2
microtubule-associated protein-2
- MMA
methyl methacrylate
- MWNT
multi-walled carbon nanotubes
- NPC
neural progenitor cells
- NSC
neural stem cell
- P3HB4HB
copolymer of 3-hydroxybutyrate and 4-hydroxybutyrate
- PANi
polyaniline
- PBCA
poly(butyl cyanoacrylate)
- PD
Parkinson's disease
- PEG
polyethylene glycol
- PHA
polyhydroxyalkanoate
- PHB
poly(3-hydroxybutyrate)
- PHBHHx
copolymer of 3-hydroxybutyrate and 3-hydroxyhexanoate
- PHDCA
polyhexadecylcyanoacrylate
- PLA
poly(lactic acid)
- PLO
poly-l-ornithine
- PLGA
poly(lactic-co-glycolic acid)
- PLLA
poly-l-lactide
- PMMAAA
copolymer of MMA and AA
- PNS
peripheral nervous system
- PPG
poly(propylene glycol)
- pVA
Poly(vinyl alcohol)
- RES
reticuloendothelial system
- SWNT
single-walled carbon nanotubes
Contributor Information
Surya K. Mallapragada, Email: suryakm@iastate.edu.
Howard E. Gendelman, Email: hegendel@unmc.edu.
References
- 1.Gendelman H, Mosley L, Boska MD, McMillan J. The promise of nanoneuromedicine. Nanomedicine. 2014;9:171–6. doi: 10.2217/nnm.14.17. [DOI] [PubMed] [Google Scholar]
- 2.Wootla B, Denic A, Warrington AE, Rodriguez M. Need for a paradigm shift in therapeutic approaches to CNS injury. Expert Rev Neurother. 2012;12:409–20. doi: 10.1586/ern.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries HE, Kooij G, Frenkel D, Georgopoulos S, Monsonego A, Janigro D. Inflammatory events at blood-brain barrier in neuroin-flammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia. 2012;53(Suppl 6):45–52. doi: 10.1111/j.1528-1167.2012.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graves D, Vernino S. Immunotherapies in neurologic disorders. Med Clin North Am. 2012;96:497–523. doi: 10.1016/j.mcna.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Kanwar JR, Sriramoju B, Kanwar RK. Neurological disorders and therapeutics targeted to surmount the blood-brain barrier. Int J Nanomedicine. 2012;7:3259–78. doi: 10.2147/IJN.S30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg GA. Neuroinflammatory disease. IBC meeting on neuroin-flammatory disease: research and treatment strategies. London, UK, 17 and 18 September 1996. Mol Med Today. 1997;3:12–3. doi: 10.1016/S1357-4310(96)30097-X. [DOI] [PubMed] [Google Scholar]
- 8.Elman LB, Houghton DJ, Wu GF, Hurtig HI, Markowitz CE, McCluskey L. Palliative care in amyotrophic lateral sclerosis, Parkinson's disease, and multiple sclerosis. J Palliat Med. 2007;10:433–57. doi: 10.1089/jpm.2006.9978. [DOI] [PubMed] [Google Scholar]
- 9.Nowacek A, Kosloski LM, Gendelman HE. Neurodegenerative disorders and nanoformulated drug development. Nanomedicine (Lond) 2009;4:541–55. doi: 10.2217/nnm.09.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imaizumi Y, Okano H. Modeling human neurological disorders with induced pluripotent stem cells. J Neurochem. 2014;129:388–99. doi: 10.1111/jnc.12625. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki H, Choong CJ, Yasuda T. The promises of stem cells: stem cell therapy for movement disorders. Parkinsonism Relat Disord. 2014;20(Suppl 1):S128–31. doi: 10.1016/S1353-8020(13)70031-2. [DOI] [PubMed] [Google Scholar]
- 12.Kang K, Kim MH, Park M, Choi IS. Neurons on nanotopographies: behavioral responses and biological implications. J Nanosci Nanotechnol. 2014;14:513–21. doi: 10.1166/jnn.2014.8764. [DOI] [PubMed] [Google Scholar]
- 13.Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J, et al. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010;40:385–403. doi: 10.1016/j.ejps.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar JR, Sun X, Punj V, Sriramoju B, Mohan RR, Zhou SF, et al. Nanoparticles in the treatment and diagnosis of neurological disorders: untamed dragon with fire power to heal. Nanomedicine. 2012;8:399–414. doi: 10.1016/j.nano.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Chen X. Applications for site-directed molecular imaging agents coupled with drug delivery potential. Expert Opin Drug Deliv. 2009;6:745–68. doi: 10.1517/17425240902889751. [DOI] [PubMed] [Google Scholar]
- 16.Lopac SK, Torres MP, Wilson-Welder JH, Wannemuehler MJ, Narasimhan B. Effect of polymer chemistry and fabrication method on protein release and stability from polyanhydride microspheres. J Biomed Mater Res B Appl Biomater. 2009;91:938–47. doi: 10.1002/jbm.b.31478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres MP, Vogel BM, Narasimhan B, Mallapragada SK. Synthesis and characterization of novel polyanhydrides with tailored erosion mechanisms. J Biomed Mater Res A. 2006;76:102–10. doi: 10.1002/jbm.a.30510. [DOI] [PubMed] [Google Scholar]
- 18.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles) Brain Res. 1995;674:171–4. doi: 10.1016/0006-8993(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 19.Grislain L, Couvreur P, Lenaerts V, Roland M, Deprez-Decampeneere D, Speiser P. Pharmacokinetics and distribution of a biodegradable drug darrier. Int J Pharm. 1983;15:335–45. [Google Scholar]
- 20.Kreuter J, Petrov VE, Kharkevich DA, Alyautdin RN. Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood-brain barrier using surfactant-coated nanoparticles. J Control Release. 1997;49:81–7. [Google Scholar]
- 21.Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16:1564–9. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- 22.Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer. 2004;109:759–67. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- 23.Alyautdin RN, Petrov VE, Langer K, Berthold A, Kharkevich DA, Kreuter J. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharm Res. 1997;14:325–8. doi: 10.1023/a:1012098005098. [DOI] [PubMed] [Google Scholar]
- 24.Alyautdin RN, Tezikov EB, Ramge P, Kharkevich DA, Begley DJ, Kreuter J. Significant entry of tubocurarine into the brain of rats by adsorption to polysorbate 80-coated polybutylcyanoacrylate nanopar-ticles: an in situ brain perfusion study. J Microencapsul. 1998;15:67–74. doi: 10.3109/02652049809006836. [DOI] [PubMed] [Google Scholar]
- 25.Alyautdin R, Khalin I, Nafeeza MI, Haron MH, Kuznetsov D. Nanoscale drug delivery systems and the blood-brain barrier. Int J Nanomedicine. 2014;9:795–811. doi: 10.2147/IJN.S52236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. discussion 16. [DOI] [PubMed] [Google Scholar]
- 27.Gelperina S, Maksimenko O, Khalansky A, Vanchugova L, Shipulo E, Abbasova K, et al. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur J Pharm Biopharm. 2010;74:157–63. doi: 10.1016/j.ejpb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV, et al. Targeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. J Control Release. 2007;122:1–9. doi: 10.1016/j.jconrel.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Zhang C, Li J, Fan L, Jiang X, Chen J, et al. Brain delivery of NAP with PEG-PLGA nanoparticles modified with phage display peptides. Pharm Res. 2013;30:1813–23. doi: 10.1007/s11095-013-1025-4. [DOI] [PubMed] [Google Scholar]
- 30.Rao KS, Reddy MK, Horning JL, Labhasetwar V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29:4429–38. doi: 10.1016/j.biomaterials.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wohlfart S, Khalansky AS, Gelperina S, Maksimenko O, Bernreuther C, Glatzel M, et al. Efficient chemotherapy of rat glioblastoma using doxorubicin-loaded PLGA nanoparticles with different stabilizers. PLoS One. 2011;6:e19121. doi: 10.1371/journal.pone.0019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamada JA, Langer R. Erosion kinetics of hydrolytically degradable polymers. Proc Natl Acad Sci U S A. 1993;90:552–6. doi: 10.1073/pnas.90.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Burkersroda F, Schedl L, Gopferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–31. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 34.Budhian A, Siegel SJ, Winey KI. Controlling the in vitro release profiles for a system of haloperidol-loaded PLGA nanoparticles. Int J Pharm. 2008;346:151–9. doi: 10.1016/j.ijpharm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Kashi TS, Eskandarion S, Esfandyari-Manesh M, Marashi SM, Samadi N, Fatemi SM, et al. Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method. Int J Nanomedicine. 2012;7:221–34. doi: 10.2147/IJN.S27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musumeci T, Ventura CA, Giannone I, Ruozi B, Montenegro L, Pignatello R, et al. PLA/PLGA nanoparticles for sustained release of docetaxel. Int J Pharm. 2006;325:172–9. doi: 10.1016/j.ijpharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Challa P, Epstein DL, Yuan F. Controlled release of ethacrynic acid from poly(lactide-co-glycolide) films for glaucoma treatment. Biomaterials. 2004;25:4279–85. doi: 10.1016/j.biomaterials.2003.10.075. [DOI] [PubMed] [Google Scholar]
- 38.Sadat T, Kashi J, Eskandarion S, Esfandyari-Manesh M, Mahmoud S, Marashi A, et al. Improved drug loading and antibacterial activity on minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method. Int J Nanomedicine. 2012;7:221–34. doi: 10.2147/IJN.S27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeifer BA, Burdick JA, Langer R. Formulation and surface modification of poly(ester-anhydride) micro- and nanospheres. Biomaterials. 2005;26:117–24. doi: 10.1016/j.biomaterials.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Determan AS, Wilson JH, Kipper MJ, Wannemuehler MJ, Narasimhan B. Protein stability in the presence of polymer degradation products: consequences for controlled release formulations. Biomaterials. 2006;27:3312–20. doi: 10.1016/j.biomaterials.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 41.Rosen HB, Chang J, Wnek GE, Linhardt RJ, Langer R. Bioerodible polyanhydrides for controlled drug delivery. Biomaterials. 1983;4:131–3. doi: 10.1016/0142-9612(83)90054-6. [DOI] [PubMed] [Google Scholar]
- 42.Wu MP, Tamada JA, Brem H, Langer R. In-Vivo Versus in-Vitro Degradation of Controlled-Release Polymers for Intracranial Surgical Therapy. J Biomed Mater Res. 1994;28:387–95. doi: 10.1002/jbm.820280314. [DOI] [PubMed] [Google Scholar]
- 43.Howard MA, III, Gross A, Grady MS, Langer RS, Mathiowitz E, Winn HR, et al. Intracerebral drug delivery in rats with lesion-induced memory deficits. J Neurosurg. 1989;71:105–12. doi: 10.3171/jns.1989.71.1.0105. [DOI] [PubMed] [Google Scholar]
- 44.Brem H, Kader A, Epstein JI, Tamargo RJ, Domb A, Langer R, et al. Biocompatibility of a biodegradable, controlled-release polymer in the rabbit brain. Sel Cancer Ther. 1989;5:55–65. doi: 10.1089/sct.1989.5.55. [DOI] [PubMed] [Google Scholar]
- 45.Jampel HD, Koya P, Leong K, Quigley HA. In vitro release of hydrophobic drugs from polyanhydride disks. Ophthalmic Surg. 1991;22:676–80. [PubMed] [Google Scholar]
- 46.Lesniak MS, Upadhyay U, Goodwin R, Tyler B, Brem H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Cancer Res. 2005;25:3825–32. [PMC free article] [PubMed] [Google Scholar]
- 47.Domb AJ, Rock M, Schwartz J, Perkin C, Yipchuk G, Broxup B, et al. Metabolic disposition and elimination studies of a radiolabelled biodegradable polymeric implant in the rat brain. Biomaterials. 1994;15:681–8. doi: 10.1016/0142-9612(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 48.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experiance. Ann Surg Oncol. 2008;15:2887–93. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 49.Tabata Y, Langer R. Polyanhydride microspheres that display near-constant release of water-soluble model drug compounds. Pharm Res. 1993;10:391–9. doi: 10.1023/a:1018988222324. [DOI] [PubMed] [Google Scholar]
- 50.Jain JP, Modi S, Kumar N. Hydroxy fatty acid based polyanhydride as drug delivery system: synthesis, characterization, in vitro degradation, drug release, and biocompatibility. J Biomed Mater Res. 2008;84A:740–52. doi: 10.1002/jbm.a.31456. [DOI] [PubMed] [Google Scholar]
- 51.Shieh L, Tamada J, Chen I, Pang J, Domb A, Langer R. Erosion of a new family of biodegradable polyanhydrides. J Biomed Mater Res. 1994;28:1465–75. doi: 10.1002/jbm.820281212. [DOI] [PubMed] [Google Scholar]
- 52.Gao J, Niklason L, Zhao XM, Langer R. Surface modification of polyanhydride microspheres. J Pharm Sci. 1998;87:246–8. doi: 10.1021/js970284u. [DOI] [PubMed] [Google Scholar]
- 53.Chavez-Santoscoy AV, Roychoudhury R, Pohl NLB, Wannemuehler MJ, Narasimhan B, Ramer-Tait AE. Tailoring the Immune Response by Targeting C-type Lectin Receptors on Alveolar Macrophages using “Pathogen-like” Amphiphilic Polyanhydride Nanoparticles. Biomaterials. 2012;33:4762–72. doi: 10.1016/j.biomaterials.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 54.Donbrow M, Samoelov Y. Controlled release of tripelennamine and other drugs dispersed in ethyl cellulose PEG films [proceedings] J Pharm Pharmacol. 1976;(28 Suppl):23P. [PubMed] [Google Scholar]
- 55.Meyskens FL, Jr, Graham V, Chvapil M, Dorr RT, Alberts DS, Surwit EA. A phase I trial of beta-all-trans-retinoic acid delivered via a collagen sponge and a cervical cap for mild or moderate intraepithelial cervical neoplasia. J Natl Cancer Inst. 1983;71:921–5. [PubMed] [Google Scholar]
- 56.Kawashima Y, Handa T, Kasai A, Takenaka H, Lin SY, Ando Y. Novel method for the preparation of controlled-release theophylline granules coated with a polyelectrolyte complex of sodium polyphosphate-chitosan. J Pharm Sci. 1985;74:264–8. doi: 10.1002/jps.2600740308. [DOI] [PubMed] [Google Scholar]
- 57.Ta HT, Dass CR, Dunstan DE. Injectable chitosan hydrogels for localised cancer therapy. J Control Release. 2008;126:205–16. doi: 10.1016/j.jconrel.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 58.Shamji MF, Hwang P, Bullock RW, Adams SB, Nettles DL, Setton LA. Release and activity of anti-TNFalpha therapeutics from injectable chitosan preparations for local drug delivery. J Biomed Mater Res B Appl Biomater. 2009;90:319–26. doi: 10.1002/jbm.b.31289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohta T, Tani A, Kimbara K, Kawai F. A novel nicotinoprotein aldehyde dehydrogenase involved in polyethylene glycol degradation. Appl Microbiol Biotechnol. 2005;68:639–46. doi: 10.1007/s00253-005-1936-z. [DOI] [PubMed] [Google Scholar]
- 60.Kawai F. Microbial degradation of polyethers. Appl Microbiol Biotechnol. 2002;58:30–8. doi: 10.1007/s00253-001-0850-2. [DOI] [PubMed] [Google Scholar]
- 61.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karatas H, Aktas Y, Gursoy-Ozdemir Y, Bodur E, Yemisci M, Caban S, et al. A nanomedicine transports a peptide caspase-3 inhibitor across the blood-brain barrier and provides neuroprotection. J Neurosci. 2009;29:13761–9. doi: 10.1523/JNEUROSCI.4246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JY, Choi WI, Kim YH, Tae G. Brain-targeted delivery of protein using chitosan- and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials. 2013;34:1170–8. doi: 10.1016/j.biomaterials.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 64.Shelke NB, Aminabhavi TM. Synthesis and characterization of novel poly (sebacic anhydride-co-Pluronic F68/F127) biopolymeric micro-spheres for the controlled release of nifedipine. Int J Pharm. 2007;345:51–8. doi: 10.1016/j.ijpharm.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 65.Hou S, McCauley LK, Ma PX. Synthesis and erosion properties of PEG-containing polyanhydrides. Macromol Biosci. 2007;7:620–8. doi: 10.1002/mabi.200600256. [DOI] [PubMed] [Google Scholar]
- 66.Gao K, Jiang X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoa-crylate nanoparticles. Int J Pharm. 2006;310:213–9. doi: 10.1016/j.ijpharm.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 67.Calvo P, Gouritin B, Chacun H, Desmaele D, D'Angelo J, Noel JP, et al. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res. 2001;18:1157–66. doi: 10.1023/a:1010931127745. [DOI] [PubMed] [Google Scholar]
- 68.Ogawara KI, Furumoto K, Takakura Y, Hashida M, Higaki K, Kimura T. Surface hydrophobicity of particles is not necessarily the most important determinant in their in vivo disposition after intravenous administration in rats. J Control Release. 2001;77:191–8. doi: 10.1016/s0168-3659(01)00468-0. [DOI] [PubMed] [Google Scholar]
- 69.Kim HR, Andrieux K, Gil S, Taverna M, Chacun H, Desmaele D, et al. Translocation of poly(ehtylene glycol-co-hexadecyl)cyanoacrylate nanoparticles into rat brain endothelial cells: role of apolipoproteins in receptor-mediated endocytosis. Biomacromolecules. 2007;8:793–9. doi: 10.1021/bm060711a. [DOI] [PubMed] [Google Scholar]
- 70.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 71.Olivier JC. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2:108–19. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 73.Kreuter J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB) J Microencapsul. 2013;30:49–54. doi: 10.3109/02652048.2012.692491. [DOI] [PubMed] [Google Scholar]
- 74.Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005;94:1187–95. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- 75.Shah L, Yadav S, Amiji M. Nanotechnology for CNS delivery of bio-therapeutic agents. Drug Deliv Transl Res. 2013;3:336–51. doi: 10.1007/s13346-013-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voigt N, Henrich-Noack P, Kockentiedt S, Hintz W, Tomas J, Sabel BA. Surfactants, not size or zeta-potential influence blood-brain barrier passage of polymeric nanoparticles. Eur J Pharm Biopharm. 2014;87:19–29. doi: 10.1016/j.ejpb.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 77.Lacasse FX, Filion MC, Phillips NC, Escher E, McMullen JN, Hildgen P. Influence of surface properties at biodegradable microsphere surfaces: Effects on plasma protein adsorption and phagocytosis. Pharm Res. 1998;15:312–7. doi: 10.1023/a:1011935222652. [DOI] [PubMed] [Google Scholar]
- 78.Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm Res. 2008;25:55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang XL, He HY, Yen C, Ho W, Lee LJ. A biodegradable, immunoprotective, dual nanoporous capsule for cell-based therapies. Biomaterials. 2008;29:4253–9. doi: 10.1016/j.biomaterials.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 80.Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release. 2012;161:264–73. doi: 10.1016/j.jconrel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 81.Uchegbu F, Siew A. Nanomedicines and Nanodiagnostics come of Age. J Pharm Sci. 2013;102:305–10. doi: 10.1002/jps.23377. [DOI] [PubMed] [Google Scholar]
- 82.Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, et al. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target. 2002;10:317–25. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 83.Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, et al. Covalent linkage of apolipoprotein E to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317:1246–53. doi: 10.1124/jpet.105.097139. [DOI] [PubMed] [Google Scholar]
- 84.Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB) Eur J Pharm Biopharm. 2009;71:251–6. doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 85.Aktas Y, Yemisci M, Andrieux K, Gursoy RN, Alonso MJ, Fernandez-Megia E, et al. Development and brain delivery of chitosan-PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconjug Chem. 2005;16:1503–11. doi: 10.1021/bc050217o. [DOI] [PubMed] [Google Scholar]
- 86.Skarlatos S, Yoshikawa T, Pardridge WM. Transport of [-125I]transferrin through the rat blood-brain barrier. Brain Res. 1995;683:164–71. doi: 10.1016/0006-8993(95)00363-u. [DOI] [PubMed] [Google Scholar]
- 87.Hu K, Shi Y, Jiang W, Han J, Huang S, Jiang X. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson's disease. Int J Pharm. 2011;415:273–83. doi: 10.1016/j.ijpharm.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 88.Tosi G, Badiali L, Ruozi B, Vergoni AV, Bondioli L, Ferrari A, et al. Can leptin-derived sequence-modified nanoparticles be suitable tools for brain delivery? Nanomedicine. 2012;7:365–82. doi: 10.2217/nnm.11.98. [DOI] [PubMed] [Google Scholar]
- 89.Pan W, Kastin AJ. Entry of EGF into brain is rapid and saturable. Peptides. 1999;20:1091–8. doi: 10.1016/s0196-9781(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 90.Gaillard PJ, Visser CC, de Boer AG. Targeted delivery across the blood-brain barrier. Expert Opin Drug Deliv. 2005;2:299–309. doi: 10.1517/17425247.2.2.299. [DOI] [PubMed] [Google Scholar]
- 91.Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB) J Drug Target. 2011;19:125–32. doi: 10.3109/10611861003734001. [DOI] [PubMed] [Google Scholar]
- 92.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis. 2010;37:48–57. doi: 10.1016/j.nbd.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 94.Liu L, Venkatraman SS, Yang YY, Guo K, Lu J, He B, et al. Polymeric micelles anchored with TAT for delivery of antibiotics across the blood-brain barrier. Pept Sci. 2008;90:617–23. doi: 10.1002/bip.20998. [DOI] [PubMed] [Google Scholar]
- 95.Tian XH, Wang ZG, Meng H, Wang YH, Feng W, Wei F, et al. TAT peptide-decorated gelatin-siloxane nanoparticles for delivery of CGRP transgene in treatment of cerebral vasospasm. Int J Nanomedicine. 2013;8:865–76. doi: 10.2147/IJN.S39951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc Natl Acad Sci U S A. 2000;97:13003–8. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rousselle C, Clair P, Smirnova M, Kolesnikov Y, Pasternak GW, Gac-Breton S, et al. Improved Brain Uptake and Pharmacological Activity of Dalargin Using a Peptide-Vector-Mediated Strategy. J Pharmacol Exp Ther. 2003;306:371–6. doi: 10.1124/jpet.102.048520. [DOI] [PubMed] [Google Scholar]
- 98.Tian XH, Wei F, Wang TX, Wang P, Lin XN, Wang J, et al. In vitro and in vivo studies on gelatin-siloxane nanoparticles conjugated with SynB peptide to increase drug delivery to the brain. Int J Nanomedicine. 2012;7:1031–41. doi: 10.2147/IJN.S26541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guarnieri D, Falanga A, Muscetti O, Tarallo R, Fusco S, Galdiero M, et al. Shuttle-mediated nanoparticle delivery to the blood-brain barrier. Small. 2013;9:853–62. doi: 10.1002/smll.201201870. [DOI] [PubMed] [Google Scholar]
- 100.Tosi G, Fano RA, Bondioli L, Badiali L, Benassi R, Rivasi F, et al. Investigation on mechanisms of glycopeptide nanoparticles for drug delivery across the blood-brain barrier. Nanomedicine. 2011;6:423–36. doi: 10.2217/nnm.11.11. [DOI] [PubMed] [Google Scholar]
- 101.Grabrucker AM, Garner CC, Boeckers TM, Bondioli L, Ruozi B, Forni F, et al. Development of novel Zn2+ loaded nanoparticles designed for cell-type targeted drug release in CNS neurons, in vito evidences. PLoS One. 2011;6:e17851. doi: 10.1371/journal.pone.0017851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Afergan E, Epstein H, Dahan R, Koroukhov N, Rohekar K, Danenberg HD, et al. Delivery of serotonin to the brain by monocytes following phagocytosis of liposomes. J Control Release. 2008;132:84–90. doi: 10.1016/j.jconrel.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 103.Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108:2827–35. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV, et al. A macrophage-nanozyme delivery system for Parkinson's disease. Bioconjug Chem. 2007;18:1498–506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kanmogne GD, Singh S, Roy U, Liu X, McMillan J, Gorantla S, et al. Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells. Int J Nanomedicine. 2012;7:2373–88. doi: 10.2147/IJN.S29454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao Y, Haney MJ, Mahajan V, Reiner BC, Dunaevsky A, Mosley RL, et al. Active targeted macrophage-mediated delivery of catalase to affected brain regions in models of Parkinson's disease. J Nanomed Nanotechnol. 2011:S4. doi: 10.4172/2157-7439.S4-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, et al. Apolipoprotein-mediated transport of nanoparticle-bound drug across the blood-brain barrier. J Drug Target. 2002;10:317–25. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 108.Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV, et al. Targeting the central nervous system: In vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. 2007;122:1–9. doi: 10.1016/j.jconrel.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 109.Snyder EY, Vescovi AL. The possibilities/perplexities of stem cells. Nat Biotechnol. 2000;18:827–8. doi: 10.1038/78428. [DOI] [PubMed] [Google Scholar]
- 110.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 112.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–89. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 114.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–7. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–31. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 116.Howk CL, Levine HA, Smiley MW, Mallapragada SK, Nilsen-Hamilton M, Sakaguchi D. A Mathematical Model for Selective Differentiation of Neural Progenitor Cells on Micropatterned Polymer Substrates. Math Biosci. 2012;238:65–79. doi: 10.1016/j.mbs.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peterson L, Oh J, Sakaguchi D, Mallapragada SK, Narasimhan B. Amphiphilic Polyanydride Films Promote Neural Stem Cell Adhesion and Differentiation. Tissue Eng. 2011;17:2533–41. doi: 10.1089/ten.TEA.2011.0095. [DOI] [PubMed] [Google Scholar]
- 118.Blong C, Ye E, Jeon CJ, Oh J, Callahan JM, Law WD, et al. Differentiation and Behavior of Human Neural Progenitor Cells on Micropatterned Substrates. J Neurosci Res. 2010;88:1445–56. doi: 10.1002/jnr.22324. [DOI] [PubMed] [Google Scholar]
- 119.Ariza C, McHugh KP, White SS, Petruk V, Sakaguchi D, Mallapragada SK. Extracellualr Matrix Proteins and Astrocyte-Derived Soluble Factors Influence the Differentiation and Proliferation of Neural Progenitor Cells. J Biomed Mater Res. 2010;94A:816–24. doi: 10.1002/jbm.a.32741. [DOI] [PubMed] [Google Scholar]
- 120.Ariza CA, Fleury AT, Tormos CJ, Petruk V, Chawla S, Oh J, et al. The influence of electric fields on hippocampal neural progenitor cells. Stem Cell Rev. 2010;6:585–600. doi: 10.1007/s12015-010-9171-0. [DOI] [PubMed] [Google Scholar]
- 121.Oh J, Recknor J, Mallapragada SK, Sakaguchi D. Soluble Factors from Neocortical Astrocytes Enhance Neuronal Differentiation of Neural Progenitor Cells from Adult Rat Hippocampus on Polymer Substrates. J Biomed Mater Res A. 2009;91:575–85. doi: 10.1002/jbm.a.32242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Recknor J, Sakaguchi D, Mallapragada SK. Growth and Differentiation of Neural Progenitor Cells on Micropatterned Polymer Substrates. Biomaterials. 2006;27:4098–108. doi: 10.1016/j.biomaterials.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 123.Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23:935–42. doi: 10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 124.Lee W, Parpura V. Chapter 6 - Carbon nanotubes as substrates/scaffolds for neural cell growth. Prog Brain Res. 2009;180:110–25. doi: 10.1016/S0079-6123(08)80006-4. [DOI] [PubMed] [Google Scholar]
- 125.Mooney E, Dockery P, Greiser U, Murphy M, Barron V. Carbon nanotubes and mesenchymal stem cells: biocompatibility, proliferation and differentiation. Nano Lett. 2008;8:2137–43. doi: 10.1021/nl073300o. [DOI] [PubMed] [Google Scholar]
- 126.Sujatha G, Sinha A, Singh S. Cells behaviour in presence of nano-scaffolds. J Biomed Nanotechnol. 2011;7:43–4. doi: 10.1166/jbn.2011.1193. [DOI] [PubMed] [Google Scholar]
- 127.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 128.Shao ZH, Wang PJ, Ni J, Li MH, Gao XL, Zhao XH. In vivo labeling of rat neural progenitor cells in subventricular zone with superparamag-netic iron oxide for tracking of magnetic resonance imaging. Zhonghua Yi Xue Za Zhi. 2013;93:807–10. [PubMed] [Google Scholar]
- 129.Chen CC, Ku MC, J DM, Lai JS, Hueng DY, Chang C. Simple SPION incubation as an efficient intracellular labeling method for tracking neural progenitor cells using MRI. PLoS One. 2013;8:e56125. doi: 10.1371/journal.pone.0056125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vande Velde G, Couillard-Despres S, Aigner L, Himmelreich U, van der Linden A. In situ labeling and imaging of endogenous neural stem cell proliferation and migration. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:663–79. doi: 10.1002/wnan.1192. [DOI] [PubMed] [Google Scholar]
- 131.Ren YJ, Zhang S, Mi R, Liu Q, Zeng X, Rao M, et al. Enhanced differentiation of human neural crest stem cells towards the Schwann cell lineage by aligned electrospun fiber matrix. Acta Biomater. 2013;9:7727–36. doi: 10.1016/j.actbio.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 132.Miyoshi S, Flexman JA, Cross DJ, Maravilla KR, Kim Y, Anzai Y, et al. Transfection of neuroprogenitor cells with iron nanoparticles for magnetic resonance imaging tracking: cell viability, differentiation, and intracellular localization. Mol Imaging Biol. 2005;7:286–95. doi: 10.1007/s11307-005-0008-1. [DOI] [PubMed] [Google Scholar]
- 133.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1:15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schiffman JD, Schauer CL. A review: Electrospinning of biopolymer nanofibers and their applications. Polym Rev. 2008;48:317–52. [Google Scholar]
- 135.Shah S, Yin PT, Uehara TM, Chueng ST, Yang L, Lee KB. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv Mater. 2014;26:3673–80. doi: 10.1002/adma.201400523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lim SH, Liu XY, Song H, Yarema KJ, Mao HQ. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials. 2010;31:9031–9. doi: 10.1016/j.biomaterials.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Du J, Tan E, Kim HJ, Zhang A, Bhattacharya R, Yarema KJ. Comparative evaluation of chitosan, cellulose acetate, and polyether-sulfone nanofiber scaffolds for neural differentiation. Carbohydr Polym. 2014;99:483–90. doi: 10.1016/j.carbpol.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Prabhakaran MP, Ghasemi-Mobarakeh L, Jin G, Ramakrishna S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J Biosci Bioeng. 2011;112:501–7. doi: 10.1016/j.jbiosc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 139.Xu XY, Li XT, Peng SW, Xiao JF, Liu C, Fang G, et al. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials. 2010;31:3967–75. doi: 10.1016/j.biomaterials.2010.01.132. [DOI] [PubMed] [Google Scholar]
- 140.Li W, Guo Y, Wang H, Shi D, Liang C, Ye Z, et al. Electrospun nanofibers immobilized with collagen for neural stem cells culture. J Mater Sci Mater Med. 2008;19:847–54. doi: 10.1007/s10856-007-3087-5. [DOI] [PubMed] [Google Scholar]
- 141.Horne MK, Nisbet DR, Forsythe JS, Parish CL. Three-dimensional nanofibrous scaffolds incorporating immobilized BDNF promote proliferation and differentiation of cortical neural stem cells. Stem Cells Dev. 2010;19:843–52. doi: 10.1089/scd.2009.0158. [DOI] [PubMed] [Google Scholar]
- 142.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–5. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 143.Callahan LA, Xie S, Barker IA, Zheng J, Reneker DH, Dove AP, et al. Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials. 2013;34:9089–95. doi: 10.1016/j.biomaterials.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 144.Kabiri M, Soleimani M, Shabani I, Futrega K, Ghaemi N, Ahvaz HH, et al. Neural differentiation of mouse embryonic stem cells on conductive nanofiber scaffolds. Biotechnol Lett. 2012;34:1357–65. doi: 10.1007/s10529-012-0889-4. [DOI] [PubMed] [Google Scholar]
- 145.Wang J, Ye R, Wei Y, Wang H, Xu X, Zhang F, et al. The effects of electrospun TSF nanofiber diameter and alignment on neuronal differentiation of human embryonic stem cells. J Biomed Mater Res A. 2012;100:632–45. doi: 10.1002/jbm.a.33291. [DOI] [PubMed] [Google Scholar]
- 146.Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnol. 2004;2:3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kiriyama A, Iga K, Shibata N. Availability of polymeric nanoparticles for specific enhanced and targeted drug delivery. Ther Deliv. 2013;4:1261–78. doi: 10.4155/tde.13.84. [DOI] [PubMed] [Google Scholar]
- 148.Xiong MH, Bao Y, Yang XZ, Zhu YH, Wang J. Delivery of antibiotics with polymeric particles. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 149.de Morais MG, Martins VG, Steffens D, Pranke P, da Costa JA. Biological applications of nanobiotechnology. J Nanosci Nanotechnol. 2014;14:1007–17. doi: 10.1166/jnn.2014.8748. [DOI] [PubMed] [Google Scholar]
- 150.Mohtaram NK, Montgomery A, Willerth SM. Biomaterial-based drug delivery systems for the controlled release of neurotrophic factors. Biomed Mater. 2013;8:022001. doi: 10.1088/1748-6041/8/2/022001. [DOI] [PubMed] [Google Scholar]
- 151.Marti ME, Sharma AD, Sakaguchi DS, Mallapragada SK. 10 - Nanomaterials for neural tissue engineering. In: Gaharwar AK, Sant S, Hancock MJ, Hacking SA, editors. Nanomaterials in Tissue Engineering. Woodhead Publishing; 2013. pp. 275–301. [Google Scholar]
- 152.Ito A, Takizawa Y, Honda H, Hata KI, Kagami H, Ueda M, et al. Tissue engineering using magnetite nanoparticles and magnetic force: Heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004;10:833–40. doi: 10.1089/1076327041348301. [DOI] [PubMed] [Google Scholar]
- 153.Ito A, Kamihira M. Tissue Engineering Using Magnetite Nanoparticles. Nanoparticles Transl Sci Med. 2011;104:355–95. doi: 10.1016/B978-0-12-416020-0.00009-7. [DOI] [PubMed] [Google Scholar]
- 154.Lui CN, Tsui YP, Ho AS, Shum DK, Chan YS, Wu CT, et al. Neural stem cells harvested from live brains by antibody-conjugated magnetic nanoparticles. Angew Chem Int Ed Engl. 2013;52:12298–302. doi: 10.1002/anie.201305482. [DOI] [PubMed] [Google Scholar]
- 155.Cheng Y, Morshed R, Cheng SH, Tobias A, Auffinger B, Wainwright DA, et al. Nanoparticle-programmed self-destructive neural stem cells for glioblastoma targeting and therapy. Small. 2013;9:4123–9. doi: 10.1002/smll.201301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Amemori T, Romanyuk N, Jendelova P, Herynek V, Turnovcova K, Prochazka P, et al. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res Ther. 2013;4:68. doi: 10.1186/scrt219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Santos T, Ferreira R, Maia J, Agasse F, Xapelli S, Cortes L, et al. Polymeric nanoparticles to control the differentiation of neural stem cells in the subventricular zone of the brain. ACS Nano. 2012;6:10463–74. doi: 10.1021/nn304541h. [DOI] [PubMed] [Google Scholar]
- 158.Li X, Liu X, Zhao W, Wen X, Zhang N. Manipulating neural-stem-cell mobilization and migration in vitro. Acta Biomater. 2012;8:2087–95. doi: 10.1016/j.actbio.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 159.Liu X, Ren X, Deng X, Huo Y, Xie J, Huang H, et al. A protein interaction network for the analysis of the neuronal differentiation of neural stem cells in response to titanium dioxide nanoparticles. Biomaterials. 2010;31:3063–70. doi: 10.1016/j.biomaterials.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 160.Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28:344–53. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 161.Liu Z, Tabakman S, Welsher K, Dai H. Carbon Nanotubes in Biology and Medicine: In vitro and in vivo Detection, Imaging and Drug Delivery. Nano Res. 2009;2:85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Heister E, Brunner EW, Dieckmann GR, Jurewicz I, Dalton AB. Are carbon nanotubes a natural solution? Applications in biology and medicine. ACS Appl Mater Interfaces. 2013;5:1870–91. doi: 10.1021/am302902d. [DOI] [PubMed] [Google Scholar]
- 163.Prasek J, Drbohlavova J, Chomoucka J, Hubalek J, Jasek O, Adam V, et al. Methods for carbon nanotubes synthesis-review. J Mater Chem. 2011;21:15872–84. [Google Scholar]
- 164.Liu F, Zhang XB, Cheng JP, Tu JP, Kong FZ, Huang WZ, et al. Preparation of short carbon nanotubes by mechanical ball milling and their hydrogen adsorption behavior. Carbon. 2003;41:2527–32. [Google Scholar]
- 165.Huang YJ, Wu HC, Tai NH, Wang TW. Carbon nanotube rope with electrical stimulation promotes the differentiation and maturity of neural stem cells. Small. 2012;8:2869–77. doi: 10.1002/smll.201200715. [DOI] [PubMed] [Google Scholar]
- 166.Moon SU, Kim J, Bokara KK, Kim JY, Khang D, Webster TJ, et al. Carbon nanotubes impregnated with subventricular zone neural progenitor cells promotes recovery from stroke. Int J Nanomedicine. 2012;7:2751–65. doi: 10.2147/IJN.S30273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park SY, Choi DS, Jin HJ, Park J, Byun KE, Lee KB, et al. Polarization-Controlled Differentiation of Human Neural Stem Cells Using Synergistic Cues from the Patterns of Carbon Nanotube Monolayer Coating. ACS Nano. 2011;5:4704–11. doi: 10.1021/nn2006128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jan E, Kotov NA. Successful differentiation of mouse neural stem cells on layer-by-layer assembled single-walled carbon nanotube composite. Nano Lett. 2007;7:1123–8. doi: 10.1021/nl0620132. [DOI] [PubMed] [Google Scholar]
- 169.Qi L, Li N, Huang R, Song Q, Wang L, Zhang Q, et al. The effects of topographical patterns and sizes on neural stem cell behavior. PLoS One. 2013;8:e59022. doi: 10.1371/journal.pone.0059022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McNamara LE, McMurray RJ, Biggs MJ, Kantawong F, Oreffo RO, Dalby MJ. Nanotopographical control of stem cell differentiation. J Tissue Eng. 2010;2010:120623. doi: 10.4061/2010/120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang K, Jung K, Ko E, Kim J, Park KI, Cho SW. Nanotopographical manipulation of focal adhesion formation for enhanced differentiation of human neural stem cells. ACS Appl Mater Interfaces. 2013;5:10529–40. doi: 10.1021/am402156f. [DOI] [PubMed] [Google Scholar]
- 172.Taylor-Pashow KM, Della Rocca J, Huxford RC, Lin W. Hybrid nanomaterials for biomedical applications. Chem Commun (Camb) 2010;46:5832–49. doi: 10.1039/c002073g. [DOI] [PubMed] [Google Scholar]
- 173.Li H, Song SI, Song GY, Kim I. Non-covalently functionalized carbon nanostructures for synthesizing carbon-based hybrid nanomaterials. J Nanosci Nanotechnol. 2014;14:1425–40. doi: 10.1166/jnn.2014.9048. [DOI] [PubMed] [Google Scholar]
- 174.Della Rocca J, Liu D, Lin W. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc Chem Res. 2011;44:957–68. doi: 10.1021/ar200028a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.He C, Lu K, Liu D, Lin W. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc. 2014;136:5181–4. doi: 10.1021/ja4098862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huxford RC, Della Rocca J, Lin WB. Metal-organic frameworks as potential drug carriers. Curr Opin Chem Biol. 2010;14:262–8. doi: 10.1016/j.cbpa.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc. 2003;125:4451–9. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 178.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 179.Park HY, Noh EH, Chung HM, Kang MJ, Kim EY, Park SP. Efficient Generation of Virus-Free iPS Cells Using Liposomal Magnetofection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Madeira C, Mendes RD, Ribeiro SC, Boura JS, Aires-Barros MR, da Silva CL, et al. Nonviral Gene Delivery to Mesenchymal Stem Cells Using Cationic Liposomes for Gene and Cell Therapy. J Biomed Biotechnol. 2010 doi: 10.1155/2010/735349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee S, Ashizawa AT, Kim KS, Falk DJ, Notterpek L. Liposomes to Target Peripheral Neurons and Schwann Cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104:29–45. doi: 10.1016/j.pharmthera.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 183.Pinzon-Daza ML, Campia I, Kopecka J, Garzon R, Ghigo D, Riganti C. Nanoparticle- and liposome-carried drugs: new strategies for active targeting and drug delivery across blood-brain barrier. Curr Drug Metab. 2013;14:625–40. doi: 10.2174/1389200211314060001. [DOI] [PubMed] [Google Scholar]
- 184.Gillies ER, Frechet JM. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 185.Kesharwani P, Jain K, Jain NK. Dendrimer as nanocarrier for drug delivery. Prog Polym Sci. 2014;39:268–307. [Google Scholar]
- 186.Benseny-Cases N, Klementieva O, Cladera J. Dendrimers antiamyloi-dogenic potential in neurodegenerative diseases. New J Chem. 2012;36:211–6. [Google Scholar]
- 187.Šebestík J, Reiniš M, Ježek J. Dendrimers in Neurodegenerative Diseases Biomedical Applications of Peptide-, Glyco- and Glycopeptide Dendrimers, and Analogous Dendrimeric Structures. Vienna: Springer; 2012. pp. 209–21. [Google Scholar]
- 188.Xu W, Ling P, Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;2013:340315. doi: 10.1155/2013/340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release. 2005;109:169–88. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 190.Onoue S, Yamada S, Chan HK. Nanodrugs: pharmacokinetics and safety. Int J Nanomedicine. 2014;9:1025–37. doi: 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int J Nanomedicine. 2014;9:4357–73. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Uchegbu IF, Siew A. Nanomedicines and nanodiagnostics come of age. J Pharm Sci. 2013;102:305–10. doi: 10.1002/jps.23377. [DOI] [PubMed] [Google Scholar]


