Abstract
Increasing oxidative stress, a major characteristic of aging, has been implicated in variety of age-related pathologies. In aging, oxidant production from several sources is increased while antioxidant enzymes, the primary lines of defense, are decreased. Repair systems, including the proteasomal degradation of damaged proteins also declines. Importantly, the adaptive response to oxidative stress declines with aging. Nrf2/EpRE signaling regulates the basal and inducible expression of many antioxidant enzymes and the proteasome. Nrf2/EpRE activity is regulated at several levels including transcription, post-translation, and interaction with other proteins. This review summarizes current studies on age-related impairment of Nrf2/EpRE function and discusses the change of Nrf2 regulatory mechanisms with aging.
Keywords: aging, antioxidant, Nrf2, oxidative stress, transcription factor
Introduction
Aerobic creatures, from unicellular organisms to human beings, are constantly exposed to oxidants and electrophiles, either from endogenous enzymatic processes or exogenous environmental pollutants. To avoid the harmful effects of these oxidative toxicants, a robust antioxidant system has evolved to maintain redox homeostasis. Oxidative stress occurs when the equilibrium of the oxidant/antioxidant balance is disrupted and tilts toward an oxidative status, which is usually accompanied by harmful effects to cell survival including lipid peroxidation and oxidative modification of proteins and nucleic acids. Indeed, oxidative stress has been implicated in various pathologies including cardiovascular and neurodegenerative diseases, cancers, diabetes, and cataract, most of which are age-related [1–4].
Disruption of the antioxidant/oxidant equilibrium is not a rare phenomenon in cells, due to the fact that the production of oxidants and the antioxidant buffering capacity always vary with metabolic, pathophysiologic changes, and environmental stress exposure. How does the organism adapt to these frequent fluctuations of redox status? Studies have found that the equilibrium of oxidant/antioxidant is maintained in a dynamic way through regulating the antioxidant levels in response to oxidative stress. Expression of antioxidant enzymes, glutamate cysteine ligase (GCL), which catalyzes the first step in glutathione (GSH) synthesis, NADPH:quinone oxidoreductase 1 (NQO-1), heme oxygenase-1 (HO-1), and many others including those that increase the reducing substrates for antioxidant enzymes, is induced in response to oxidative stimuli including both environmental toxicants and electrophiles derived from dietary antioxidants [5]. The increase in antioxidant capacity, which can be called nucleophilic tone enhances the removal of excessive oxidants and prevents further severe oxidative injury. The response of antioxidants to oxidative stress evolves as a critical defense mechanism to combat harmful effects of intrinsic and extrinsic oxidative insults, and is preserved in all organisms.
In recent decades, the most exciting discovery concerning the response to oxidative stress has been elucidation of the signaling pathway by which such responses are regulated. Central to our understanding of such regulation is the activation of nuclear factor erythroid 2 -like 2 (NFE2L2; more commonly known as Nrf2) and its interaction with Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1, Keap1. Nrf2 is a transcription factor that controls the basal and inducible expression of an array of antioxidant and detoxification enzymes including the proteasome. Along with partners, interacting proteins, and regulatory molecules, the Nrf2 signaling system has emerged as perhaps the most important cellular defense and survival pathway against oxidative stress and toxicants. Disruption of Nrf2 signaling is associated with an increased susceptibility to oxidative insults and other toxicants in humans and model organisms [6–10].
The tightly regulated nucleophilic tone becomes compromised with aging, and leads to a chronic oxidative state in old organisms [10, 11]. Studies have established that age-related oxidative damage involves an increase in oxidant production, decrease in antioxidant capacity, and less efficient activity of both the proteasome and the mitochondrial Lon protease; the net result being the accumulation of intracellular and intramitochondrial masses of oxidized and cross-linked protein aggregates [12–20]. Accumulating evidence suggest that the decline of the adaptive response of antioxidant to oxidative stimuli, especially the Nrf2/EpRE signaling system, also plays a key role in the accumulation of oxidative damage in aging [21–23].
Nrf2/EpRE signaling
As other reviews in this special issue deal with the fundamentals of Nrf2/EpRE signaling, here we provide only a very brief review of that area, in order to provide the context for our discussion of how aging affects Nrf2 activation. Moi et al. identified nuclear factor E2-related factor 2 (Nrf2) as a regulator of β-globin expression in 1994 [24]. Its function as a transcription factor to regulate the expression of antioxidant and detoxification enzymes was first reported in 1996 as an activator of the so-called antioxidant response element (ARE) in the NQO-1 promoter region and regulate its induction by β-naphthoquinone and t-butylhydroperoxide [25]. Before then, ARE had been characterized as the cis-element essential for the basal and inducible expression of many antioxidant and detoxification genes, including rat and mouse glutathione S-transferase Ya subunit (GST-Ya) [26], rat and human NQO-1 [27], and rat GST-P [28]. The cis-element was initially named as ARE because of its activation by phenolic antioxidants. Shortly thereafter it was found that most ARE inducers are actually electrophiles or function through generating H2O2 [26, 29]. Therefore, the name electrophile response element or EpRE is more accurate although the use of ARE persists.
Before exposure to electrophiles, Nrf2 interacts in the cytosol with a protein called Keap1. Keap1 interact with other proteins and plays the key role in regulating the localization of Nrf2, the degradation of Nrf2, and in sensing oxidative stimuli: 1) Through its Kelch domain, Keap1 binds to Nrf2 and sequesters it to the actin or myosin cytoskeleton; 2) Keap1 can act as an adaptor for Cullin 3-based ubiquitin ligase E3 complex that leads to the proteasomal degradation of Nrf2 [30]; 3) Some cysteine residues in the cysteine-rich intervening region, especially cys 151, 273 and cys288, are required for Nrf2 binding. Meanwhile they are extremely sensitive to oxidative and electrophilic modification. When these cysteine residues are modified, Keap1 loses its Nrf2 binding ability and Nrf2 thereby escapes from degradation.
EpRE is the Nrf2 binding cis-element present in promoter regions of many genes. It has a core sequence of TGANNNNGC required for Nrf2 binding. This so-called consensus EpRE sequence can be found in many genes, but only some of them are functional in terms of transcriptional activation through Nrf2 binding. It seems that some flanking nucleotides and nucleotides within the “NNNN” sequence also play important roles in EpRE function [31, 32]. Nrf2 binds to EpRE after forming a heterodimer with other basic leucine zipper proteins such as small Maf proteins MafG/F/K, or though other proteins, including c-Jun, Jun-B, Jun-D, Fra1, and ATF4 are also found in Nrf2-EpRE binding complexes [25, 33–36].
Oxidative stress and aging
The oxidative stress (free radical) theory of aging proposed by Denham Harman in 1956 [37] postulates that reactive oxidants generated endogenously causes cumulative oxidative damage to macromolecules resulting in the aging phenotype. This long standing theory has been challenged, modified, and expanded by many but two fundamental arguments remain: first, an imbalance of antioxidant/oxidant occurs with aging that results in the accumulation of oxidatively damaged macromolecules; and second, the accumulating oxidative damage causes a degenerative aging phenotype. Although the second point is disputed by some studies recently (see reviews [38–42]), the former point, i.e., that the antioxidant/oxidant equilibrium is disrupted and oxidatively damaged macromolecules accumulate in the elderly, is well established and accepted [38, 43].
There seems little doubt that the accumulated oxidative damage with aging is caused by either an increased production and/or decreased elimination of oxidants and electrophiles [43]. Numerous studies have shown that there is an elevation of steady state oxidant concentrations in cells and tissues from aged organism. The main sources of endogenous oxidants include the electron transport system in the mitochondria, a number of oxidoreductases including xanthine oxidase, cytochrome p450, monoamine oxidase, and nitric oxide synthase, and enzymes involved in the inflammatory and infection response to xenobiotic stimulation including NADPH oxidases [44]. The production of oxidants from these sources varies with pathophysiological situations, and a tendency to increase with age is observed for many of them. Several excellent reviews on aging and oxidant generation are available [38, 45–47]. Nonetheless, as this review is part of a special issue on Nrf2 signaling, we will focus on the changes in how aging affects cellular removal of oxidants and other electrophiles.
Adaptive responses of antioxidant enzymes to oxidative stress in aging
Another essential mechanism underlying how oxidative stress increases in age is the diminished antioxidant capacity, including lower basal antioxidant concentrations and impaired adaptive induction of antioxidants in response to oxidative stress. Here we will focus on the antioxidant enzymes for which a role of Nrf2 has been implicated in their regulation, although studies on the effect of aging on antioxidant enzymes predate the discovery of their regulation by Nrf2 by a decade or more. The Nrf2-associated antioxidant enzymes include the two subunits of glutamate-cysteine ligase (GCL) [48], which catalyze the first and committed step in de novo glutathione (GSH) biosynthesis, all three members of superoxide dismutase [49] family [50, 51], catalase [50], glucose-6-phosphate dehydrogenase (G6PDH) [51–54], some members of the peroxiredoxin (Prdx) [55, 56], glutathione peroxidase (GPx) [52], glutathione S-transferase [57] families [50–52], Sulfiredoxin [58, 59], and thioredoxin reductase [60, 61]. The regulation by Nrf2 at the transcriptional level has been shown for some of these enzymes, but in some cases the association with Nrf2 has only been demonstrated by the effect of knocking out or silencing Nrf2. Basal levels of the antioxidant enzymes are determined by genetics, diet, drugs, pathologies, and environmental stresses [62]. The reducing substrates for antioxidant enzymes, NADPH and GSH also can change with age as a result of the changes in the antioxidant enzymes, specifically G6PDH, isocitrate dehydrogenase, malic enzyme, nicotinamide nucleotide transhydrogenase (NNT), and GCL.
Dietary antioxidants, which except for vitamin E do not actually play a direct role in reducing intracellular oxidants [5], may change in aging if intake or absorption is altered. Many of these compounds or their metabolites are inducers of Nrf2 and their potential for altering the effect of aging on the Nrf2 signaling with age will be considered later.
Changes of antioxidant enzymes with aging
Antioxidant enzymes convert reactive and toxic oxidants and electrophiles into stable and less toxic or neutral molecules, and are the main first-line mechanism of maintaining redox homeostasis and defending against oxidative damage. The age-dependent change of antioxidant enzymes has been being extensively studied. Even though, great controversy exists in the literature as to whether or not these enzymes vary with aging, and increases, decreases, or no changes have been reported. Difference in results of these studies might be related to variations in species, strain, tissue, sex, and experimental design. Here age-related change of SOD, catalase, GPXs, and Prdxs are briefly summarized.
SODs
SOD catalyzes the dismutation of two superoxide anion radicals (plus two protons) into O2 and H2O2, which can then be removed by catalase, GPxs, and Prdxs. Two types of SOD exist in cells, Cu, Zn-SOD (SOD1) in the cytosol, and Mn-SOD (SOD2) in mitochondria. A third form, also containing Cu and Zn (SOD3), is found extracellularly. There are many studies on the change of SOD activity with aging, but the results reported are inconsistent, or even contradictory. Decreases in SOD1 have been observed in various tissues or cells from old human subjects in comparison with that from young adults, including skin fibroblasts [63, 64], lymphocytes [65], skeletal muscles [66], serum [67], plasma and erythrocytes [68, 69]. Animal studies also showed an age-dependent decrease in SOD1 activity in liver [70, 71], and brain [72, 73] of old rats. In contrast, no age-related differences in SOD1 activity were reported in erythrocytes [74], plasma [75], lymphocytes [76], plasma [77], and muscles [78] from human subjects of different ages, or in rat liver (21 mo vs. 6 w) [79]. In addition, some studies even showed that SOD1 activity was increased with aging in some tissues, such as mouse skeletal muscles, rat brain [80], and human plasma [81]. It remains elusive what causes the controversy on age-related change of SOD1 activity. Tissue-related differences are observed in some studies, i.e., Ji and collaborators found that SOD1 level was increased in skeletal muscle but decreased in liver of old rat [70].
SOD2 plays a critical role in production of O2·− and H2O2 in mitochondria as the leak to produce O2·− is actually kinetically unfavorable and pulled forward by SOD2 [82]. With aging, SOD2 activity was found to be decreased in skeletal muscles of mouse [83], brains, hearts, livers, and kidneys of rats [84], and Drosophila melanogaster [85]. Lu et al. found that although SOD2 activity was increased with aging in skin fibroblasts from humans of under 60 years of age, it actually decreased in later years [63]. In contrast, SOD2 activity was reportedly increased in skeletal muscles of humans [66, 78] and rats [70, 86], or not changed [76, 87] with aging.
SOD3 dismutates extracellular O2·− and, concomitantly, produces H2O2.; by so doing it is involved in regulating the availability of extracellular O2·− and appears to play an important role in controlling oxidative stress and intercellular redox signaling. Increased SOD3 with aging has been observed in the prostatic lobes [88] and renal cortex of rats [89]. In contrast, SOD3 expression was decreased in retinal pigment epithelial cells from older donors (>60 y) compared with that from young donors [90]. There is evidence that SOD3 expression was not altered by aging in the lung of mice when its level was reduced by LPS injection in the old compared to the young mice [91]. Another study also shown that basal SOD3 was decreased in the kidney cortex of old rats and failed to be induced by exercise in comparison with younger counterparts, but aortic SOD3 showed no change with exercise or age [92]. These findings suggest that the basal expression of SOD3 might change with aging in a tissue specific manner and its induction seems impaired in the old.
Catalase, GPxs, and Prdxs
Catalase dismutates H2O2 to H2O and O2, while GPxs reduce H2O2 to H2O using GSH. GPx4 can also reduce lipid hydroperoxides to their corresponding alcohols. Reports on age-related changes of catalase and GPx are conflicting. With aging, catalase activity was decreased in skin fibroblasts [63], erythrocytes [74], lymphocytes [65, 76], and skeletal muscles of human subjects [93]. Studies on animals also showed decreased catalase activity in kidneys [94] and livers of rat [95]. In contrast, increased catalase activity with aging is observed in glycolytic muscles of rat [96] and serum of elders [67]. In addition, no change of catalase activity with aging was reported in skeletal muscles [66], plasma [75] and erythrocytes of old human subjects [69]. Doria et al. proposed that the contradictory data could be due to the dynamics of catalase activity during aging [97], which showed two-phase trend in rats [70]. However, this dual-phase trend is inconsistent with evidence from a human study [65]. Age-related changes of catalase activity seem tissue dependent. For example, it was increased in the hippocampal and striate regions but decreased in the cortex and septal area in the brains of old rats [80]; and in another study, catalase activity was elevated in vestibular tissue but unchanged in vascular or sensory cochlear tissues in the ears of old rats [98].
The GPx family consists of 8 members in mammals, GPx1-4 and GPx6 containing selenium, while GPx5, GPx7, and GPx8 are non-selenium proteins [99]. The physiological localization and substrate specificity of each GPx varies, and collectively protect against a wide spectrum of oxidants, however the function of GPx6, GPx7, and GPx8 remains to be elucidated [99–101].
Age-related changes in GPx have been extensively studied, mostly by measuring the total GPx activity. An age-related decrease of GPx activity has been demonstrated in plasma [75, 102], skin fibroblasts [63], erythrocytes [74], and lymphocytes [65, 76] of humans, and livers of rats [95]. However, no age-related change of total GPx activity was reported in human erythrocytes [69] and skeletal muscles [66] and rat liver (22 mo vs. 5 mo) [71] and skeletal muscles [96]. In contrast, an age-dependent increase of GPx activity was observed in erythrocyte of older people (older than 60 y) [103] and rats [104]. Collectively, current data suggest that age-associated changes in GPx activity appear related with the specific species, strain, sex and tissue studied [105, 106]. It should be noted though that GPx activity levels include the activities of several GPxs. Due to the different location, regulation, substrate specificity, and potential different functions of individual GPxs in aging, it would be worthwhile to elucidate the age-related change of each GPx member.
GPx1 expression showed no change in the brain of old mice [107] and endothelium of skeletal muscle arteries from old rats (22 mo vs 3 mo) [108]. But a recent study showed that GPx1 protein level and activity was significantly decreased in endothelial progenitor cells (EPCs) of older subjects (72 y) in comparison with that of young subjects (24 y) [109]. Decreasing GPx1 activity with aging was also reported in rat sperm [110]. In contrast, its expression was increased with aging in the ovaries of mice [111].
An age-related change in GPx3 has been the focus of some studies. GPx3 mRNA was increased significantly in retinal pigment epithelium from old mice (24 mo vs 2 mo) [112]. On the other hand, a study from Xu et al. demonstrated that GPx3 mRNA remained unchanged while its protein level decreased with aging in kidneys of rats [113], suggesting a possible posttranslational regulation of GPx3 with age. As Gpx3 is the only GPx secreted into plasma, the serum Gpx activity should generally represent Gpx3 activity although some other isoenzymes may come from disrupted cells including GPx1 from hemolysis. There is some controversy at present concerning the change of plasma GPx activity with aging in humans, with both decreases [75] and increases [102] reported. Although the underlying reasons for these discrepancies remain unclear, differences appear to be influenced by age and sex [102, 114].
GPx4 plays a critical role in protecting membranes from oxidative damage. GPx4 activity in liver microsomal membrane of old rat was significantly higher than that of young [115]. In contrast, GPx4 activity and its protein level in the nucleus was decreased in sperm of aged rats [110]. However, an age-related change in GPx4 activity was not observed in the liver, kidney, lung, and brain of rats, compared to the total GPx activity (including GPx1, 2, and3), which was decreased in old compared with young adults [116].
Peroxiredoxin (Prdx)
Prdx is a family of peroxidases that reduce H2O2 and lipid hydroperoxides, to H2O and alcohols. To date, six mammalian isoenzymes of Prdx (Prdx1-6) have been identified. Prdxs 1 through 5 reduce H2O2 to H2O using thioredoxin, while Prdx6 can also reduce lipid hydroperoxides to corresponding alcohols using GSH [117, 118]. The oxidized forms of Prdxs are accumulated in cell or tissues with aging. In mitochondria oxidized Prdx3 increased in aged rat liver compared with that of young adults (28 mo vs 12 mo) while the reduced form remained unchanged [119]. The oxidized form of Prdx in hippocampus was lowest at 12 mo and started to increase thereafter [120, 121]. The age-related accumulation of hyperoxidized Prdx appears to be linked to the increase of oxidant production with aging; however, it may also suggest a less efficient Prdx repairing mechanism in senescent cells.
A change in total and/or active forms of Prdxs with aging has also been reported. A decreased Prdx2 protein level with aging was observed in interstitial fluid of bone marrow of rats [122] and plasma of mice [123]. A decrease in Prdx3 with aging was found in ovaries [111] and livers of rats [124]. A progressive decline in Prdx5 expression with advancing age was also identified in human nerves at different ages [125]. On the other hand, it is reported that expression levels of Prdx6 mRNA in whole lenses gradually increased in the lenses of 1 to 6 mo mice and declined thereafter [126].
GSTs
GST super family comprises multiple isoenzymes that locate differentially in cytosolic, membrane, and mitochondria [127]. The classic GSTs usually refers to the GSTs in the cytosol that are encoded by at least five distantly related gene families (alpha, mu, pi, sigma, and theta GST) [128]. GSTs transfer GSH to electrophiles and thereby play critical roles in oxidative defense and detoxification. Many studies have examined the change of GST expression and activity with aging. Table I listed the major findings from some of these studies. Age-related decrease of total GST activity was reported in lymphocytes [65], skeletal muscles [93], gastric mucosa [129] of human, and hepatic cells of rat [95, 130]. However, the age-dependent change of GST activity is not consistent and divergent results are also reported. For example, some of the GST isoenzymes, protein levels of GSTm and GSTp did not change with aging in lymphocytes [131], and total GST activity in rat liver was also unchanged with aging [71, 132]. Some studies even demonstrate an increase of GST with aging. For instance, GSTD2 was increased with aging in Drosophila [85]. Data from Table x suggest that age-related change of GST exhibit specie and tissue specific manner. In most human tissues studied, GST activity is decreased, while results become conflicting in the animal studies. In rat liver, for example, increases, decreases, or unchanged levels of GST activity have all been reported. GST expression can be influenced by many factors such as specie, sex, food, growth condition, and adaptive induction, etc. Especially, the mRNA levels of individual GST isoenzymes in rat liver exhibit different patterns of change with aging and sex [133] and this makes it even harder to explain the controversial results. Nonetheless all available information suggests that GST activity might be decreased in senescent cells, while age-related changes of individual GST isoforms remains largely unclear
Table I.
Age-related change of GST expression and activity
| Marker | Change | Cell or tissue | Species | Age | Reference |
|---|---|---|---|---|---|
| GST activity | Decrease | Skeletal muscle | Human | [93] | |
| GST activity | Decrease | Lymphocytes | Human | 50–60 y vs. 20–30 y | [65] |
| GST activity | Decrease | Gastric mucosa | Human | 19–63 y | [129] |
| GST activity | Unchanged | Erythrocytes | Human | 1 mo–63 y | [134] |
| GST activity | Decrease | Liver | Rat | 20 mo vs. 2 mo | [130] |
| GST activity | Down by 70%, recovered by safranal | Brain | Rat | 20 mo vs. 2 mo | [73] |
| GST activity | Increase | Soleus muscle | Rat | 26 mo vs. 13 mo | [135] |
| GST activity | Increase | Skeletal muscle and liver | Rat | 31 mo vs. 4 mo | [70] |
| GST activity | Decrease in cytosol, increase in mitochondria | Liver | Rat | 31 mo vs. 4 mo | [70] |
| GST activity | Decrease by 4 times | Liver | Rat | 22 mo vs. 3 mo | [95] |
| GST D2 | Increase | - | Fly | 30d vs. 3d | [85] |
| GST activity and GSTa protein | GST activity is up by 5.6 folds, GSTa protein unchanged | Liver | Rat | 21 mo vs. 1.5 mo | [79] |
| Protein of GSTa and GSTp | No change | Lymphocytes | Human | 60–80 y vs. 20–40 y | [131] |
| GST activity | No change | Liver | Rat | 26 mo vs. 6 mo | [132] |
| GSTm2 mRNA | Decreased | Ovaries | Mice | 12 mo vs. 2–9 mo | [111] |
| mRNA of GST isoenzymes | m2 higher, m4 and p1 lower in male; t2 lower and a1 higher in female, others not changed | Liver | Mice | 21 mo vs. 3 mo | [133] |
| GST activity | Unchanged | Liver | Rat | 22 mo vs. 5 mo | [71] |
GSH
GSH is the most abundant antioxidant in cells and tissues, and plays a primary role in protection against oxidative stress. Age-associated variation of GSH has been extensively studied. Maher et al. summarized the studies on variation of GSH with aging in 2005 [136]. According to these studies, total and reduced GSH concentration are markedly diminished and the disulfide form of GSH, GSSG, increased in many tissues from aged experimental animals and human subjects in comparison with young adults [136]. In follow up studies, GSH concentration was found to decrease in liver (22 mo vs. 5 mo) [71], brain (20 mo vs. 2 mo) [73] of rat, carotid artery of Rhesus macaques (20 y vs. 10 y) [137], and human lymphocytes (50–60 y vs. 20–30 y) [65]. This age-dependent decrease of GSH is also supported by several in vivo human studies with noninvasive methods. Using a noninvasive NMR method, Emir et al. found that GSH concentrations in the brains of older people (70 y old) decreased by 30% compared to young (20 y) [138]. A recent study using stable deuterium-labeled glycine found that both GSH concentration and its synthesis rate in RBC of elderly (60–75 y) were decreased by ~50% compared with that of adult (20–40 y) [139].
In contrast, there are also reports that GSH concentration may not be changed or may be increased with aging in some tissues or cells. For instance, GSH concentration was unchanged in in the plasma from old human subjects [129, 140] and in the liver of aged mice (24 mo vs. 6 mo) [141], and it was increased in muscles of aged rat (26 mo vs. 13 mo) [135].
Taken together, these data suggest that there is a general age-dependent decrease of GSH with exceptions in some tissues. In the short term, GSH homeostasis is well maintained in cells through a precisely regulated system involving induction of enzymes for GSH synthesis. Due to a shift to more oxidative status with aging, GSH consumption, including its reaction with H2O2, lipid hydroperoxides, electrophiles, and its binding to protein to form protein-mixed disulfides increases [142]. Meanwhile the degradation rate of GSH is also increased in the elderly due to increased γ-glutamyl transpeptidase activity. Besides the increased GSH consumption and degradation, another potential explanation for the age-dependent decrease in GSH is that the adaptive response of GSH synthesis system to oxidative stress may be deficient or impaired in old organisms. It is well established that oxidative stress or disturbance of GSH homeostasis usually causes the induction of an adaptive response that increases GSH production [143].
Accumulating evidence suggests that disruption of this homeostasis is the cause of the age-related decline in GSH concentration. Gould reported that the adaptive response of GSH synthesis to cigarette smoke was significantly impaired in the lung of old mice. GSH in the extracellular lining fluid of the lung, which reflects intracellular synthesis [144] was increased 6 fold in response to cigarette smoke in the young (2 mo) but only 2 fold in the old mice (26 mo), and this made old mice more susceptible to oxidative damage. Sekhar et al. recently also shown that GSH synthesis rate in RBC of elderly (60–75 y) was decreased by 50% compared with that of adult (20–40 y) [139]. The KM of GCL for its substrates, glutamate and cysteine, is significantly increased during aging due to age-related accumulation of homocysteine, which decreases the affinity between GCL and its substrates [145] [141]. This would adversely affect the ability for rapid GSH biosynthesis, especially under stressful conditions. The age-dependent decline of adaptive response of GSH to oxidative stressors is closely related with the decreased induction of GCL in response to oxidative stress with aging (see next section).
GCL
GCL plays a critical role in maintaining GSH homeostasis and its expression level is usually proportional to GSH concentration [146, 147]. GCL consists of a catalytic (GCLC) and a modifier (GCLM) subunit. Although they form a 1:1 complex, a higher ratio of GCLM to GCLC favors the formation of the higher activity heterodimer [148]. The oxidant-induced expression of both subunits is finely regulated through a complex mechanism that has been being extensively studied and demonstrated that these genes are co-regulated through their TRE and EpRE elements [35, 149–152].
Considering the age-dependent variation of GSH level and the critical role of GCL in regulating GSH homeostasis, many studies have compared the expression of GCLC and GCLM in elderly to that of young animals. In 2000, Liu and Choi first reported that both the mRNA and protein levels of both GCLC and GCLM were decreased in the livers, lungs, and kidneys of aged rats along with the activity of GCL in erythrocytes (24 mo vs. 6 mo) [153]. The age-dependent decline of both GCLC and GCLM was also observed in the livers of old rat (24–28 mo vs. 2–5 mo) [147]. Recent evidence suggests however, that the changes from basal expression of GCLC and GCLM may diverge in aging. A decrease of GCLC but unchanged GCLM expression with aging was observed in the skeletal muscles of rat [135], on the other hand, a decrease in GCLM and unchanged GCLC expression was demonstrated in the brains of old rats [154]. Yuan et al. examined the change of GCL with aging in the kidneys of rats and found that although GCL activity was significantly decreased, the basal mRNA and protein levels of both GCLC and GCLM were not changed in the old (21 mo) compared with young (2 mo) rats [155]. Age-dependent change of GCLM was neither observed in mice lymphocytes [156]. We recently reported that the basal expression levels of both GCLC and GCLM were significantly higher in the lung, liver, and cerebellum of middle-aged mice (21 mo) in comparison with young adult (6 mo), along with a higher expression level of other phase II genes and nuclear Nrf2 level [157]. Sachedeva et al. also observed an increased GCLM expression in retinal pigment epithelium of middle-aged mice (15 mo vs. 2 mo) [158]. A study using Rhesus monkeys demonstrated that GCLC was decreased in carotid arteries and VSMC of old (20 y) compared to that of young (10 y) while GCLM expression was not changed [137]. In summary, the age-related variation of basal level of GCLC and GCLM is not consistent. It remains unclear whether the divergence in GCL expression with aging is related with species, tissues, or frailty, or how this occurs in humans.
Induction of GCL is a critical mechanism of adaptive responses to oxidative stress and its variation with aging should have a significant impact on age-related susceptibility to oxidative insults. Suh et al. examined the induction of GCL in response to lipoic acid, and found that GCLC expression was induced by about 2 times in the liver of old rat (24–28 mo), while GCLM was not induced [147]. Unfortunately GCL induction in the young was not reported and thus, an age-related comparison was not made. Yuan et al. evaluated the GCL induction in kidney in response to resveratrol, GCLM mRNA was induced in both young (2 mo) and old (21 mo) but not in middle aged (12 mo) rat, and the GCLC mRNA was only induced in the old [155]. When looking at the age-related susceptibility to nanoparticle toxicity, we compared the induction of GCL expression in response to nanoparticle [157]. In the lung, liver, and cerebellum of young mice (6 mo), the mRNA and protein levels of both GCLC and GCLM were induced significantly (1.5–2 fold), while in the tissues of old (21 mo), the induction of GCL was completely lost. The loss of GCL induction in response to nPM was associated with decline of Nrf2 expression. Ungvari et al. observed that basal GCLC mRNA level in aorta segment remained stable from 3 mo to 18 mo and started to decrease from 24 mo, and it was induced in response to H2O2 in young (3 mo) but not old (24 mo) rat [159]. A recent publication from Sachedeva et al. also found that the induction of GCLM in retinal pigment epithelium in response to sodium iodate declined in middle-aged mice (15 mo vs. 2 mo) [158].
HO-1
Heme oxygenase-1 (HO-1) catalyzes the oxidation of heme to biliverdin, free iron and carbon monoxide. Numerous studies have shown that HO-1 plays critical roles in antioxidant defense and various pathophysiologic processes, including age-related pathophysiologic changes. As a result, the effect of aging on HO-1 regulation has been a research focus for decades.
Table III summarizes the major findings from past decades on the change of HO-1 expression with aging, including its basal and inducible expression upon stimuli. Increased basal HO-1 levels, marked by its, mRNA or protein levels, or its enzymatic activity, is observed with aging in various tissues such as liver, lung, brain, kidney, spleen, hippocampus, and cerebellum in most of these studies. In contrast, some reported no change or a decrease. For example, HO-1 expression is not changed in the liver of Wistar rat (24 mo vs. 1.3 mo) [162], and the ear skin (20 mo vs. 2 mo) [163], cerebral cortex of mice (23–24 mo vs. 5–6 mo) [164], cochlea (11 mo vs. 3 mo) [165], and macrophages from mice (3,6,18,21 mo) [166]. HO-1 is decreased in the hippocampus (20 mo vs. 2 mo) [167], spinal cord and astrocytes (13 mo vs. 1.5 mo), and aorta of rat (24 mo vs. 3 mo) [159]. It should be noted that most of studies used rodents as a model and study of human subjects was rare. Hirose and colleagues examined the HO-1 protein level in autopsied brain of 31 human subjects from 3–84 y old, and found that HO-1 increased with aging in the hippocampus and cerebellum [168]. In addition, the age-related increase in HO-1 expression could be abrogated. The study by Rechelhoff et al. showed that HO-1 level was increased in the kidney of old rats, but vitamin E treatment blocked the increase [169]. Another study also found that dietary restriction reduced higher HO-1 expression in the brains of old rats [170]. Nonetheless reports on age-dependent changes in basal HO-1 expression have generated divergent results, and this may reflect a complex species- and tissue-dependent difference in HO-1 expression. For example, HO-1 level is increased in the liver but not changed in the spleen of old mice (24 mo vs. 6 mo) [171]. Variation in what age was considered as young and old is another possible explanation for the divergence of basal HO-1 level with aging, as activities may not change linearly with age or change direction from young to middle to old age.
Table III.
Age-dependence change of HO-1
| Marker | Change | Cell or Tissue | Specie | Age | Reference |
|---|---|---|---|---|---|
| HO-1 | Activity increased, response to decreasing cellular heme levels not changed | Liver | Rat | 24 mo vs. 2 mo | [179] |
| HO-1, HO-2 | Liver HO-1 is increased in old mice, but not induced by iron, spleen ho1 is not changed by age or iron, HO2 unchanged | Liver, spleen, | Mice | 24 mo vs. 6 mo | [171] |
| HO-1 | Homogenates: Basal HO-1 is increased in old, but induction is significantly decreased by heat stress (5 vs. 2 fold) Hepatocytes: higher HO-1 protein but no induction in old |
Liver and hepatocytes | F344 rat | 24 mo vs. 6 mo | [172] |
| HO-1 | Basal increased | Liver | F344 Rat, f | 30–32 mo vs. 3–4 mo | [180] |
| HO-1 activity | Unchanged | Liver | Wistar rat, m | 24 mo vs. 1,3 mo | [162] |
| HO-1 activity | Induction declined in response to paraquat | Liver | SAM mice | 12,17 mo vs. 2,7 mo | [173] |
| HO-1 expression | Increased, vitamin E reduced HO-1 expression | kidney | Rat | 22 mo vs. 12 mo | [169] |
| HO-1 | Increased | Hypothalamus (Astrocytes) | SD rat | 24 mo vs. 2 mo | [181] |
| HO-1 | Increased, reduced by food restriction | Brain | Rat | 24 mo vs. 2 mo | [170] |
| HO-1 | Increased | Kidney | F344 Rat | 24 mo vs. 6 mo | [182] |
| HO-1 mRNA | Increased | Hippocampus and cerebellum | Rat | 28 mo vs. 6 mo | [183] |
| HO-1 protein | Increased | Hippocampus and cerebellum | Human | 3–84 y | [168] |
| HO-1 | Increased, HIF1a increased | Liver | F344 Rat | 6,12,18,24 mo | [184] |
| HO-1 | Increased, induced by sulforaphane | Spleen lymphocytes | Mice | 19–21 mo vs. 2–4 mo | [156] |
| HO-1 | Decreased, declined response to hypoxia | Hippocampus | Rat | 20 mo vs. 2 mo | [167] |
| HO-1 | Unchanged, responsive to ischemia | Ear skin | Mice | 20 mo vs. 2 mo | [163] |
| HO-1 mRNA and protein | Increased in 15 mo, unchanged in 26 mo, Response blunted in 26 mo | Gastro intestine | Mice | 2 mo vs. 15 mo, 26 mo | [175] |
| HO-1 mRNA | Basal increased, response to ethanol reduced in 6 mo and lost in 18 mo | Liver | Rat | 2 mo, 6 mo,18 mo | [178] |
| HO-1 | Basal higher, impaired induction to sodium iodate | Retinal pigment epithelium | Mice | 15 mo vs. 2 mo | [158] |
| HO-1 | Basal level no change, induction by injury is decline in mRNA but not in protein level, basal HIF1a increased, induction decreased | Cerebral cortex | Mice | 23–24 mo vs. 5–6 mo | [164] |
| HO-1 protein | Decreased induction to hypoxia | Carotid body | Wistar rat | 24 mo vs. 2 mo | [174] |
| HO-1 | Basal unchanged, LPS induction reduced in lung of 18 mo, lost in 21 mo AM | Microphage | Mice | 18 mo vs. 3 mo, 21–14 mo vs. 6 mo | [166] |
| HO-1 | Basal increased | Liver | Rat | 24 mo vs. 2 mo | [185] |
| HO-1 | Reduced induction | Macrophage | Mice | 12 mo vs. 2 mo | [186] |
| HO-1 | Decreased | Cochleae | Mice | 11 mo vs. 3,6 mo | [165] |
| HO-1 | Basal decreased, induction declined in response to H2O2 and glucose | Aorta | Rat | 24 mo vs. 3 mo | [159] |
| HO-1 | Basal and induction not changed | Kidney | Mice | 16 mo vs. 6 mo | [177] |
| HO-1 | Increased mRNA | Hippocampus | Rat | 30, 33 mo vs. 6 mo | [187] |
| HO-1 | Basal increased, induction to nPM declined | Lung, liver, brain | Mice | 21 mo vs. 6 mo | [157] |
| HO-1 | Induction declined | Endothelial cells | Rat | 24 mo vs. 3 mo | [188] |
| HO-1 | Higher in middle aged than old | Substantianigra and striatum | SD Rat | 22 mo vs. 2–4 mo | [189] |
It has been well documented that HO-1 is induced by various stressors including oxidative stress as a key component of the adaptive mechanism against oxidative and electrophilic toxicity. Given the involvement of oxidative stress and HO-1 in aging-related pathophysiological changes, there has been intensive interest in HO-1 induction change in old organisms (Table III). Impaired HO-1 induction has been frequently observed in various tissues of old rodents and by variety stimulators. Compared with that in young rodents, HO-1 induction declined in the liver of old rodents in response to iron [171], heat stress [172], and paraquat [173]; in hippocampus by hypoxia [167]; in aorta of old rats by H2O2 and glucose [159]; in the carotid body [174], the gastrointestinal tract [175], and retinal pigment epithelium of old mice [158]. It should be noted that the age-related decline of HO-1 induction in rodents occurs independently of the basal HO-1 level. The age-related decline of HO-1 induction was further supported by a recent study on human senescent cells. Lima and colleagues found that HO-1 is induced by curcumin in primary young human skin fibroblast, but HO-1 in senescent cells, which was already relatively high, was not further induced by curcumin [176].
The extent of HO-1 induction may be related to age. For instance, Nath et al. showed that HO-1 induction was similar in the kidney of young (6 mo) and middle-aged (16 mo) mice [177]. Patriarca et al. found that HO-1 induction in response to ethanol began to decrease in the liver of 6-month-old and was lost in that of 24-month-old rats [178]. Ito et al. also showed that LPS-stimulated HO-1 induction was reduced in alveolar macrophages from 18 mo mice, and was completely lost in macrophages from 21 mo mice [166]. In addition, the decline of age-related induction appears to be tissue-independent. HO-1 induction by iron was reduced in the liver but not changed in the spleen of old rats [171]. Another study also showed that HO-1 induction by sulforaphane was similar in the spleen lymphocytes from young and old mice (21 mo vs. 4 mo) [156]. Collectively these results indicate that HO-1 induction in response to oxidative stressors is impaired in older organisms.
NQO-1
NAD(P)H:quinone oxidodreductase (NQO-1) is a flavoenzyme that catalyzes the two-electron reduction of various quinones and aromatic compounds by utilizing NAD(P)H as an electron donor. It is an important antioxidant enzyme in maintaining the cellular redox state. As a critical part of the cellular defense mechanism, NQO-1 expression level is induced in response to electrophilic and/or oxidative stress due to exposure to chemicals or endogenous quinones.
Many studies have investigated the change of NQO-1 expression in old animals in order to understand how it is involved in age-related oxidative damage, but results on the basal expression of NQO-1 diverge. Fu and colleagues demonstrated that NQO-1 expression was increased in the liver of 24 mo mice compared with that of 3 mo [133]. We recently reported that NQO-1 mRNA level was significantly higher in the lung, liver, and cerebellum of middle-aged mice (21 mo) in comparison with young adults (6 mo) [157]. Inconsistent with these results, basal NQO-1 level was increased in memory T cells from old mice [156]. A very recent report also found it was increased in retinal pigment epithelium of middle-aged mice (15 mo vs. 3 mo) [158]. In contrast, Ungvari and collaborators reported that NQO-1 expression was decreased in the aorta from 24 mo rats (vs. 3 mo) [159], though they did not see a change of NQO-1 expression in the carotid artery and vascular smooth muscle cells (VSMC) of old Rhesus macaques (20 y vs. 10 y) [137]. Another study also showed a decrease of NQO-1 expression in astrocytes of old mice [190]. Due to the limited data, it remains unclear whether the difference in the age-dependent change of the basal expression of NQO-1 is due to species, tissues, cell types, or aging phases.
Variation of induction of NQO-1 in response to stressors with aging may play a significant role in the antioxidant defense capacity in the elderly. Ungvari et al. studied NQO-1 induction in the aorta in response to H2O2 and glucose, and found that NQO-1 induction declined in aorta from old rats (24 mo vs. 3 mo) [159]. The same group also demonstrated that although NQO-1 expression did not change, its induction in response to H2O2 was blunted in the carotid artery and VSMC from old (20 y) in comparison with young Rhesus macaques (10 y) [137]. We also found that compared with young adult mice, NQO-1 induction in response to nanoparticles was abrogated in the liver, lung, and cerebellum of middle aged mice [157]. The decline of NQO-1 induction was also observed in the frontal cortex and cerebellum of rats (24 mo vs. 4 mo) [191] and retinal pigment epithelium of middle-aged mice (15 mo vs. 3 mo) [158]. An age-related decline of NQO-1 induction may occur in tissue specific manner. For example, NQO-1 was not induced by toluene in the frontal cortex and cerebellum of old mice, but induced in hippocampus [191]. NQO-1 was also induced similarly by sulforaphane in spleen lymphocytes from young and old mice (21 mo vs. 4 mo) [156]. Nonetheless, current data support that NQO-1 induction is impaired in various tissues of aged organisms.
Nrf2-EpRE signaling in the decline of the antioxidant response in aging
Both the basal and inducible expression levels (in response to stressors) of the antioxidant enzymes described above are regulated, at least in part, through activation of Nrf2/EpRE signaling. It is been well established that Nrf2 is the master transcription factor that controls the basal and inducible expression of hundreds of antioxidant and detoxifying enzymes. While there is quite a bit of diversity in the effect of age on the basal expression of these enzymes, there is general consensus that the ability to induce these enzymes by electrophiles declines with age. Accumulating data suggest that this age-dependent decline in the antioxidant enzyme response is caused by declining efficiency of Nrf2/EpRE signaling (Table V).
Table V.
Age-dependent change of basal Nrf2 and its activation
| Nrf2/EpRE activity | Change | Cell or tissue | Specie | Age | Reference |
|---|---|---|---|---|---|
| EpRE activity | Increased | Head, abdomen | Drosophilae | 30 d vs. 6 d | [85] |
| Nrf2/EpRE activity | Decreased | Intestinal stem cells | Drosophilae | 30 d vs. 3 d | [193] |
| Nrf2 mRNA and EpRE signaling | Nrf2 mRNA not changed, induction of EpRE signaling decreased | Whole | Drosophilae | 10 d–50 d | [195] |
| Nrf2 binding and protein | Decreased, activation by H2O2 blunted | Carotid arteries, VSMC | Rhesus macaques | 20 y vs. 10 y | [137] |
| Nuclear Nrf2 | Decreased, but restored by EGCG | Spinal cord and astrocyte | Mice | 1,5,13 mo | [190] |
| Nrf2 | Basal nuclear Nrf2 unchanged, increased by exercise | Proximal renal tubule | F344 rat | 21 mo vs. 3 mo | [194] |
| Nrf2 | Basal nuclear Nrf2 decreased, induction declined in response to H2O2 and glucose | Aorta | F344Xbrown Norway | 24 mo vs. 3 mo | [159] |
| Nrf2 | Basal increased, induction and activation to nPM declined | Lung, liver, brain | Mice | 21 mo vs. 6 mo | [157] |
| Nrf2 | Decreased | Liver | Rat | 18–24 mo vs. 2,12 mo | [192] |
| Nrf2 | Both total and nuclear Nrf2 decreased, lipoic acid increased Nuclear Nrf2 and GCL | Liver | Rat | 24–28 mo vs. 2–5 mo | [147] |
| Nrf2 expression | Increased | Spleen lymphocytes | Mice | 19–21 mo vs. 2–4 mo | [156] |
Aging-related changes in Nrf2/EpRE activity
Many studies have studied the change of Nrf2/EpRE activity, including its nuclear level and the binding to EpRE motif in older organisms. In a pioneering study, Suh and colleagues showed that both the total and nuclear Nrf2 protein levels were significantly lower, accompanied by a reduced level of GCL, in the liver of 24–28 mo in comparison with 2–5 mo rats [147]. Shih and Yen later confirmed this finding, showing that Nrf2 expression and its target genes exhibited an age-dependent decrease in rats [192]. Another study also found that nuclear Nrf2 was decreased in the aorta from old rats (24 mo vs. 3 mo) [159], along with reduced GCLC, NQO-1, and HO-1 levels. Additional work from the same group using carotid arteries and VSMC from aged Rhesus monkey (20 y vs. 10y) also saw decreased Nrf2 levels and decreased binding to EpRE [137]. In contrast, Kim et al. found that the expression level of Nrf2 and its target genes was significantly increased in spleen lymphocytes from old mice [156]. We also showed that total and nuclear Nrf2 was increased in the liver, lung, and cerebellum of 21 mo mice in comparison with that of 6 mo, simultaneously with increased expression of its target genes [157]. It is unclear if these discrepancies are caused by species difference. According to current data, age-related change of basal Nrf2/EpRE signaling exhibit a tissue or cell specific manner. For example, Nrf2/EpRE activity was increased in the head and abdomen of old Drosophila (30 d vs. 6 d) [85], but it was decreased in the intestinal stem cells from flies at the same age (30 d vs. 3 d) [193]. Another study showed that nuclear Nrf2 was not changed in the proximal renal tubule of 24m compared with that of 3 mo rats [194]. Collectively, current evidence suggests that basal Nrf2 levels and activity change with age, but the direction may be different dependent on species, tissues, and cell types. Obviously more systematic studies are required to explain these divergent findings.
We studied Nrf2/EpRE activation in response to nanoparticles and found that although the basal Nrf2 level was increased in 21 mo vs. 6 mo mice, Nrf2/EpRE activation and the induction of Nrf2-target genes were lost in the liver, lung, and cerebellum in the middle-aged mice (21 mo), compared with that of young adults (6 mo) [157]. Ungvari and collaborators also showed that Nrf2 activation and the induction of its targeted genes declined in response to H2O2 and glucose in the aorta of old rats [159], and in the carotid arteries and VSMC from old Rhesus monkeys [137]. It is worthwhile to note that the decline of Nrf2/EpRE induction with aging is independent of the age-related change of basal Nrf2/EpRE activity. In contrast, Suh et al. reported that age-dependent decrease of Nrf2/EpRE activity could be restored. They showed that lipoic acid activated Nrf2 in the liver of old rat and induced GCLC expression [147]. Another study on Nrf2 activation by exercise also demonstrated that nuclear Nrf2 was increased in the proximal renal tubules of old rats (24 mo vs. 3 mo) [194]. Gounder et al. also showed that Nrf2 content was reduced in the myocardium of old mice (23 mo vs. 2 mo), but moderate exercise could recover aging-impaired Nrf2 signaling [23]. On the other hand, a recent study showed that Keap1 knockout partially restored the induction of NQO-1, whose induction was impaired in the retinal pigment epithelium of middle-aged mice (15 mo), while the induction of HO-1 and GCLM was not recovered [158]. Unfortunately change of total and nuclear Nrf2 protein after Keap1 knockout was not reported so it is impossible to know if the Nrf2 stability or nuclear translocation is affected by aging. Nonetheless these controversial results suggest the necessity of further studies concerning impairment of Nrf2/EpRE function with aging.
In addition, proteins other than Nrf2 itself might also cause age-related loss of Nrf2/EpRE function. Rahman et al. [195] found no change of basal mRNA expression of Nrf2 and its target genes in flies with aging, but the induction in response to oxidative stress was lost in the old flies. Overexpression of the small Maf protein, Maf-S, restored the age-related decline of induction of Nrf2 target genes (GSTD1, GCLC, GCLM) in response to oxidants; however, as the basal expression level of the MafS did not decline with age, the decline of Nrf2 signaling function may involve activation of MafS rather than its expression or the activation of Nrf2, the change of which with aging was not determined.
Aging phenotypes of Nrf2 and Keap1 knockout/knockdown mice
Nrf2 and/or Keap1 knockout mice provide good in vivo models to examine the roles of Nrf2 and its regulated genes in aging. Nrf2 knockout mice exhibit higher sensitivity to oxidants and electrophiles [196–198], more susceptible to carcinogenesis [199–201], and other pathologies caused by environmental insults [202]. Fewer studies however, have focused on aging. The hearing ability of Nrf2 null mice was significantly more impaired than that of age-matched wild-type mice at 6 and 11 mo, and that the numbers of hair cells and spiral ganglion cells were remarkably reduced in Nrf2-knockout mice [203]. Serum testosterone level and its production in Leydig cells in Nrf2−/− mice was reduced significantly as early as 8 mo, while this only occurs by old age (21–24 mo) in wild-type mice [204]. In addition, Nrf2 knock out mice are more susceptible to skin aging caused by ultraviolet B. [205]. This limited evidence suggests that Nrf2 deficiency may result in at least some phenotypes of aging.
Keap1 knockout is lethal to mice by weaning age and therefore conditional Keap1-null mice that allow partial disruption of Keap1 expression later in life were created. Keap1 null mice show higher levels of antioxidant enzymes and are more resistant to acute toxicity; however, an increased mortality in 2-yr-old mice is observed when Keap1 level is decreased to less than 50% of normal [206]. This evidence suggests that although transient induction of Nrf2 signaling and its regulated genes is beneficial, constitutive high level Nrf2 activation may be disadvantageous to long-term survival. Alternatively, other functions of Keap1 may play a vital role. Obviously further study is required to resolve these issues.
The Nrf2 regulatory network and its change in aging
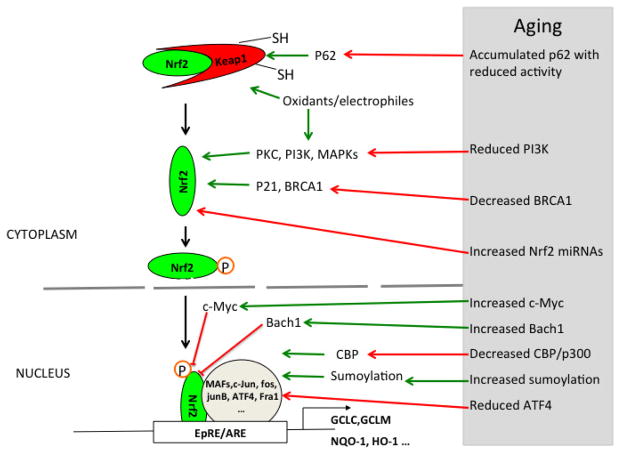
Nrf2/EpRE signaling system is regulated at several levels. The main line of regulation is formed by the core components Keap1, Nrf2, and Nrf2 partner proteins that bind to the EpRE cis-element. The complex formed by transcription factors Nrf2 and its partner (small Mafs, c-Jun, ATF4, etc.) forms the positive regulation arm, leading to enhanced expression of antioxidants genes, and also the expression of Nrf2 repressors such as Keap1, Bach1, and β-CrTP that form the negative arm of the main feedback loop of Nrf2/EpRE signaling. These negative regulators either interact with Nrf2 cause its degradation or compete with Nrf2 for EpRE binding, or repress its transactivation. The next level of Nrf2 regulation is provided by post-translational modification such as phosphorylation and sumoylation, which regulate the intracellular distribution, activity, and degradation of Nrf2 or its partners. The third level of Nrf2 signaling regulation occurs as a result of various associations of Nrf2 with other proteins.
Activation of Nrf2-EpRE signaling
Under resting conditions, most Nrf2 is rapidly degraded with a half-life of less than 30 min through its interaction with Keap1. Upon exposure to oxidative and electrophilic stressors, the redox sensitive cysteine residues in Keap1 are modified, allowing Nrf2 to dissociate from Nrf2-Keap1 complex and escape degradation. Dissociated Nrf2 is then translocated into the nucleus, forms heterodimers with other transcription factors, binds to EpRE, and enhances gene transcription. Dissociation of the 19S regulator from the 26S proteasomal complex during stress (e.g. oxidative stress) also appears to transiently increase ‘free’ Nrf2 levels because Nrf2 degradation by the ATP/Ubiquitin-26S proteasomal system is temporarily lost [207]. This process, catalyzed by Ecm29 and HSP70, appears to increase Nrf2 translocation to the nucleus, binding to EpRE, and transcription of target genes.
In the past two decades, there has been extensive investigation concerning the role of Nrf2 in pathologies, and protection against oxidative insults. It is now well known that Nrf2 plays an essential role in maintaining redox homeostasis and protecting against oxidative insults (see other reviews in this issue), and that Nrf2 dysregulation is implicated in various oxidative stress-related diseases including cardiovascular diseases [208], neurodegenerative diseases [209], pulmonary diseases [210], cancers [211], and other pathologies [212].
Nrf2 phosphorylation
Studies have reported that phosphorylation is required for Nrf2 activation and target gene induction [213]. The involvement of protein kinases PKCδ [214–216], PI3K [215, 217–219] in Nrf2 dissociation and nuclear transportation in response to diverse oxidative stressors has been observed in many systems. While reports on roles of MAPKs (ERK1/2, JNK, p38MAPK) in Nrf2 activity regulation are controversial (see review [220]), accumulating evidence suggest that ERK1/2 increases Nrf2 stability and activity [221–226], while others found that inhibition of ERK1/2 did not abolish Nrf2 signaling [215, 227–229]. The controversy might be due to difference in species, cell types, and stressors [230]. P38MAPK is implicated in the induction of Nrf2/EpRE-regulated genes [215, 228, 230–238], but its direct role in Nrf2 activation is less clear. Keum et al. reported that p38MAPK negatively regulated Nrf2 activation [239], but this was not supported by others who found that activation of p38MAPK was required for Nrf2 nuclear transportation and activation [223, 225, 228]. In addition, many studies have also shown the involvement of JNK in Nrf2 nuclear translocation [240–243].
There is evidence suggesting that Nrf2 phosphorylation on tyrosine may play a role in the export of Nrf2 from the nucleus and its degradation [244–251]. This “post induction response” of Nrf2 signaling is apparently controlled by the PI3K/AKT-GSK-3β-Fyn axis.
Age-related change of PI3K and PKC activity
The possible involvement of PI3K in both the nuclear translocation and export of Nrf2 protein makes it an interesting target for studies of aging in relation to Nrf2 signaling. Most studies on Nrf2 phosphorylation have been made of in cell lines and direct evidence of whether these findings hold true in senescent cells and aging process is limited although Shih and Yen found that the age-dependent decrease of Nrf2 protein and its target genes was associated with reduced activation of mitogen-activated protein kinase cascade in liver of old rat (18 and 24 mo vs. 2 and 12 mo) [192].
An age-related change of PI3K protein and activity has been reported in many studies. Decreased PI3K/AKT signaling was observed in skeletal muscle of old mice [252] and in hepatocytes of old rat [253]. It is appeared that the age-related decrease in PI3K signaling could be due to a decrease of its p85α subunit, as reported in cardiac muscles of old mice [254] and rats [255]. This was confirmed in both mice and human pancreatic tissues, and in liver, lung, and kidney of mice [256]. In addition, PI3K/AKT signaling was also decreased in brains (24 mo vs. 6 mo) [257] and hippocampus of old mice [258], and in macrophages from elderly individuals [259].
In contrast, an age-related increase in PI3K signaling was reported in some studies. Increased PI3K signaling has been reported in the peripheral blood mononuclear cells [260] and CD4+ lymphocytes [261] from old human donors. Animal studies also showed that PI3K signaling was increased in skeletal muscle (26 mo vs. 6 mo) [262] and heart of old rats [263], and macrophages of old mice [264]. Majumdar et al. showed an increased phosphorylation of p85α and Akt in the colonic mucosa of old compared with that of young rats [265]. A recent study by Tomobe et al. evaluated age-related change of Nrf2 signaling in a mouse model of accelerated aging (SAMP8) [266] and found that total and nuclear Nrf2 protein in liver of 10-month-old SAMP8 mice were decreased compared to that of normal age-matched SAMR1 mice, associated with a decreased AKT phosphorylation.
These results suggest that age-associated alterations in PI3K/Akt signaling may be tissue specific, and this may imply a different effect on the regulation of Nrf2/EpRE activity with aging. In addition, change of p85α protein level with aging appears to be an important underlying mechanism associated with age-related variation of PI3K signaling. Nonetheless, whether and how age-related change of PI3K signaling affects Nrf2/EpRE function with aging is unclear and should be elucidated with further studies.
PKC has many isoenzymes that differ significantly in regulation, specificity and location. PKCδ has been implicated in Nrf2 translocation [214, 215], but few, if any, of the several studies that have determined the effect of aging on PKC have looked specifically at PKCδ.
Nrf2 sumoylation and the age-related change of sumoylation
Protein sumoylation occurs when proteins are covalently bound to the small ubiquitin-related modifier (SUMO) family proteins (SUMO-1, -2/3). Sumoylation-mediated protein modification is involved in various cellular processes through modulation of protein localization, regulation of transcription, and protein-protein interactions. Ramani et al. first reported that two potential SUMO binding sites existed in the b-ZIP domain of Nrf2 and Nrf2 sumoylation by SUMO-1 was required for Nrf2/MafG interaction and the EpRE activation in rat hepatic stellate cells, evidenced by the abrogation of Nrf2/EpRE activity with the mutation of Nrf2 sumoylation sites or the knockout of SUMO-1 [267]. Nrf2 sumoylation and its role in promoting Nrf2-MafG interaction and Nrf2/EpRE activation was further confirmed in hepatocytes and macrophages derived from mice treated with endotoxin [268]. Nrf2 sumoylation by SUMO2/3 was also detected but does not seem to be involved in the regulation of Nrf2 activity. Malloy et al. investigated the sumoylation of Nrf2 and found that Nrf2 was a target for SUMO-1 and SUMO-2/3 [269], As2O3, an inducer of Nrf2, increased SUMO-2-conjugated Nrf2 in 1–4 h after exposure. Interestingly, they found that polysumoylated Nrf2 was simultaneously ubiquinated by poly-SUMO-specific E3 ubiquitin ligase RNF4, resulting in proteasomal Nrf2 degradation and decreased steady state Nrf2 level in the nucleus. However, this sumoylation-mediated Nrf2 degradation only contributes about 30% of the basal and inducible Nrf2/EpRE activity. Based on this study, polysumoylation of Nrf2 by SUMO-2/3 seems to cause Nrf2 degradation. It is unclear whether Nrf2 sumoylated by SUMO-1 and that by SUMO-2/3 have a different fate or function.
Interestingly, sumoylation is redox regulated and the SUMO conjugation activity is reduced under oxidative stress [270]. SUMO conjugating enzymes, such as SUMO E1 subunit Uba2 and the E2-conjugating enzyme Ubc9, could be inhibited by direct and reversible oxidative modification through the formation of (a) disulfide bond (s) involving the catalytic cysteines [270]. SUMO protease SENP1 is also redox regulated and its desumoylation activity increases under oxidative stress condition [271]. In addition, SUMO-3 is negatively regulated under oxidative stress [272]. However, there are studies suggest a controversy result that sumoylation of some proteins is increased upon oxidative stress [273, 274]. Given these controversies about the potential role of sumoylation in Nrf2 regulation, and about the redox regulation of sumoylation, further study on its role in the Nrf2/EpRE system in response to oxidative stress is guaranteed.
Protein sumoylation is implicated in cell senescence, demonstrated by the findings that sumoylation overexpression increases while its deficiency reduces senescence [275]; however age-dependent variation of protein sumoylation is less well characterized. An increase in protein sumoylation with aging has been observed in the spleens [276] and hearts [277] of rats (25 mo vs. 3 mo). Yang reported that protein sumoylation and SUMO-3 were increased in the hypothalamus of aged mice (25 mo vs. 7 mo) [278]. A recent study from Sapir et al. showed that the sumoylation of HMG-CoA synthase in C. elegans increased with aging, and this might be due to an age-related decrease of the activity of ULP-4 small ubiquitin-like modifier protease [279]. This evidence suggests that protein sumoylation may be increased with age. Unfortunately, the effect of sumoylation on age-related change of Nrf2/EpRE signaling pathway, including on Nrf2 itself, is unclear. Considering the controversy effect of oxidative stress on sumoylation system and the pro-oxidative status of aging, this issue needs to be elucidated.
Other proteins regulating Nrf2/EpRE signaling and their potential role in aging
Besides the aforementioned regulatory mechanisms, evidence shows that many other proteins regulate Nrf2/EpRE signaling. These proteins either associate with Nrf2 to regulate its stability, or compete with Nrf2 for EpRE binding. Here we briefly summarized these proteins based on their positive or negative effects on Nrf2/EpRE activity.
Proteins positively regulating Nrf2/EpRE signaling
P21 WAF1/Cip1) protein
Cyclin-dependent kinase inhibitor p21 mediates multiple cellular processes including cell cycle arrest, senescence, apoptosis, and protection against oxidative stress [280]. The expression of p21 is up regulated in response to oxidative stress. Chen et al. found that p21 deficiency reduced the basal and inducible Nrf2 level and its target genes. Further state-of the art experiments demonstrate that p21 could directly interact with Nrf2 through its KRR motif. Since p21 competitively bind to the DLG and ETCG motifs in Nrf2, the same motifs bound by KEAP1, Nrf2- KEAP1 interaction is reduced, so as KEAP1-mediated Nrf2 degradation, in other words, Nrf2 level and Nrf2/EpRE signaling is increased [281]. Up-regulation of Nrf2 by p21 might be a feedback mechanism since Nrf2 activation could reduce p21 and revert p21-mediated growth inhibition [282]. Buitrago-Molina reported that p21 knockout induced Nrf2 and its target genes via Sestrin 2, which is increased and activates Nrf2 through p65-mediated KEAP1 degradation [283].
P21 is a direct participant in regulating genes involved in cell senescence and aging [284–288]. Enomoto et al. demonstrated that there was an age-related increase of p21 protein level in human corneal endothelial cells [289]. On the other hand, Simon et al. found that total p21 expression was unchanged while p21 in the nucleus declines in liver of aged rat compared with that of young [290]. In agreement, Song et al. showed no significant difference in the expression level of p21 in corneal tissues from donors with different ages [291]. These limited data indicate that p21 expression level might not change with aging, but that its translocation to the nucleus may decline. Currently little is known on whether Nrf2/EpRE signaling is differently influenced by p21 in old organisms.
Breast cancer susceptibility gene 1 (BRCA1)
BRCA1 is a well-established tumor suppressor that is implicated in maintaining genome integrity through DNA repair processes. Recent evidence suggests that BRCA1 is also involved in defense against oxidative stress [292]. Bae et al. first reported that BRCA1 overexpression up-regulated the expression of antioxidant enzymes including NQO-1 and increased defense against oxidative stress while BRCA1 deficiency conferred sensitivity to oxidant damage [293]. They also demonstrated that BRCA1 stimulated Nrf2/EpRE signaling. Later Gorrini et al. found that BRCA1 could regulate Nrf2-EpRE signaling by physically interacting with Nrf2 and promoting its stability and activation. BRCA1-deficiency resulted in down regulation of Nrf2-regulated antioxidant enzymes in mouse primary mammary epithelial cells [294]. In addition to its direct interaction with Nrf2, BRCA1 might also regulate Nrf2/EpRE signaling via interacting with other proteins. For example, BRCA1 induces p21 protein [295] [296], interacts with CBP/p300 [297], c-Myc [298], which are involved in Nrf2 regulation (see corresponding parts of current review). Taken together, BRCA1 appears to positively regulate Nrf2/EpRE signaling and participate in antioxidant defense. The emerging role of BRCA1 as a regulator of Nrf2/EpRE signaling, especially how it interacts with other Nrf2 regulators, however, needs to be further elucidated.
Although BRCA1 mutation has been extensively studied, due to its implication in breast cancer, less is known about BRCA1 expression level in aging. Pan et al. demonstrated that BRCA1 expression was reduced in oocytes obtained from middle-aged mice (18 mo vs. 3 mo) [299]. Another study also found that the mRNA and phosphorylated BRCA1 were decreased in the primordial follicles of aged rats (15 mo vs. 1 mo) [300]. Titus et al. measured the BRCA1 protein in oocytes from mice and women at different ages and found its level declined with age [301]. In contrast, higher level of BRCA1 was found in Alzheimer’s disease [302].
CREB binding protein (CBP)
CBP is a transcription co-activator with intrinsic histone acetyltransferase activity that is involved in chromatin opening [303, 304]. CBP is involved in the regulation of the activity of a large number of general and cell-specific transcription factors. CBP can interact with numerous transcription factors of different classes [305, 306]. The promiscuous binding characteristics together with reports of requirement of CBP binding for sufficient function of many transcription factors, have led to the suggestion that competing for limited amounts of CBP and related molecules would provide a coordinating mechanism whereby various intracellular signaling pathways integrate to accomplish the appropriate transcriptional activity [306]. This model has been supported by results from the recently reported CBP/p300 gene-deleted mice [57]. CBP is recruited to Nrf2/EpRE complex through association with Nrf2 via its NEH4 and NEh5 domain, and regulates transcription [303, 307]. Sun et al. demonstrated that CBP could directly bind and acetylates the lysines of Nrf2 in response to arsenite-induced oxidative stress [308]. Acetylation was partially involved in Nrf2 activity. Further studies found that acetylation enhanced Nrf2 binding ability to EpRE instead of affecting Nrf2 stability [308].
Decreased histone acetylase activity of CBP/P300 is observed in liver, muscle, and testes of aged mice [309], motor-neurons [310], cerebral cortex and hippocampus of aged rats [311]. However, some studies suggest that CBP level remains relatively stable in the brain, lung, spleen, and heart of old mice compared to young [309], and in hippocampus of aged rats (26–28 mo vs. 6 mo) [312]. Radak et al. also did not find the change of CBP in skeletal muscle of elderly human (62 y vs. 26–30 y) [313]. It remains unclear whether this inconsistency is due to differences in species, tissues or aging phase. Nonetheless, a decline of CBP activity may cause dysregulation of Nrf2 signaling and its target genes in aging. Shenvi et al. found that the typical EpRE activity in GCLC was diminished during aging because of the absence of CBP and less deacetylation in livers of old rats [314], this caused the binding of Nrf2 to an alternative EpRE site located −2.2 kb downstream from the normally active EpRE binding site. However, the transcription activity of this alternative EpRE-Nrf2 complex was not sufficient for GCLC transcription [314]. A recent report showed that there might be a competition for CBP binding between Nrf2 and NF-κB [315]. Considering the increase of NF-κB level with aging [316], CBP binding with Nrf2 would be decreased.
Sequestosome-1 (SQSTM1, p62)
P62 was initially discovered as an atypical protein kinase C (PKC)-interacting protein. Now it has been recognized that p62 interacts with several signaling pathways and is a crucial molecule in a myriad of cellular functions [317]. P62 was identified as a positive regulator of Nrf2/EpRE signaling when Liu et al. screened for Nrf2-associated genes [318]. Overexpression of p62 increased nuclear Nrf2 and Nrf2/EpRE activity and NQO-1 induction, Nrf2 transcription and total Nrf2 seems not affected [318]. P62 is an ubiquitin binding protein acting as a cargo receptor for autophagic degradation of ubiquitinated proteins. It is increased in response to various stimuli and interacts with Keap1, and causes Keap1 accumulation and subsequent autophagic degradation, resulting in the inhibition of the binding of Keap1 and Nrf2, and inhibition of Keap1-mediated Nrf2 degradation [319]. Jain et al. further showed that p62 interacted with the Kelch-repeat domain of Keap1, which disrupted the interaction of Keap1 with Nrf2. Because p62 is polymeric, the interaction between KEAP1 and p62 leads to KEAP1 aggregation with p62 bodies and its subsequent autophagic degradation. Interestingly p62 is a target of Nrf2/EpRE signaling and its expression is increased along with Nrf2 activation in response to oxidative stress, this forms a positive feedback loop of Nrf2 regulation [320]. Recently it is reported that arsenic activates Nrf2 through a non-canonical pathway in which it causes accumulation of p62 while tert-butylhydroquinone and sulforaphane activate Nrf2 through the canonical pathway [321]. Indeed accumulating evidence suggests this autophagy deficiency/p62 accumulation dependent pathway is important in maintaining the integrity of Nrf2-Keap1 system [322–324].
In contrast with other Nrf2/Keap1 interacting proteins, the age-dependent changes in p62 have been extensively studied. With aging, p62 protein level is increased in various cells and tissues such as cardiomyocytes (24–26 mo vs. 3–4 mo) [325] and hippocampus of mice [326], kidney (24 mo vs. 3 mo) [327], islet (24 mo vs. 4 mo) [328], and osteocytes of rats (24 mo vs. 3 mo) [329]. An aged-related increase of p62 could be reduced by calorie restriction [330] or exercise [331]. Bartlett et al. found that increase of p62 protein with aging in neural tissues of flies was not due to increased transcription, as its mRNA level was decreased [332]. The age-dependent increase of p62 protein occurs as an accumulation within inclusions, which form from aggregation of p62 and ubiquitinated proteins, due to declining autophagic activity [332]. The degradation of p62 is mainly through autophagy [333]. Impaired autophagy has been observed in various tissues and cells from senescent organisms [334], including islet cells (24 mo vs. 4 mo) [328], extraocular muscles [335], and osteocytes (24 mo vs. 3 mo) of rats [329]. Therefore, it would seem reasonable to expect an increase of p62 accumulation with aging; however, p62 is markedly more aggregated with aging [332]. In other words, although total p62 protein is increased in senescent cells, its activity is likely to be decreased. Thus, the subsequent p62-mediated Nrf2-EpRE regulatory pathway could be down-regulated with aging.
Transcriptional regulator activating transcription factor 4 (ATF4)
ATF4 is a member of the ATF/cAMP-response element-binding (CREB) group of the bZIP transcription factor family, it has been involved in the regulation of genes involved in amino acid transport, GSH homeostasis, and oxidative defense [336]. A role for AFT4 in Nrf2-EpRE activation has been suggested [337]. Using the yeast two-hybrid system, He and collaborators identified ATF4 as a heterodimerization partner of Nrf2 that was involved in the activation of EpRE signaling of HO-1 [338]. ATF4 expression is induced by diverse stressors, including oxidative stressors [338–340], through Nrf2/EpRE signaling [341]. In addition, ATF4 expression is also regulated at the translational level through the change of phosphorylation of eIF2α, which binds to the 5′- untranslated region of the ATF4 mRNA [342, 343].
Elevation of ATF4 is related to longevity in several aging models [344]. ATF4 expression is reduced in various tissues of old mice (18 mo vs. 1 mo) due to the decline of eIF2α phosphorylation, which is associated with a higher level of GADD34, a subunit of eIF2α phosphatase [345]. Similarly a decline of eIF2α phosphorylation was observed in aged mouse cerebral cortex (22–24 mo vs. 10w) [346], suggesting a reduced ATF4 expression. Drummond et al. showed that eIF2α phosphorylation and the nuclear ATF4 level in skeletal muscles were increased in the young but not old subjects (68 y vs. 28 y) following resistant exercise [347].
Proteins negatively regulating Nrf2/EpRE signaling
Keap1
KEAP1 was identified as an Nrf2 repressor in 1999 when Itoh et al. screened Nrf2 associated proteins using a yeast two-hybrid assay [348]. Keap1 acts both as an anchor for cytosolic Nrf2 by binding to cytoplasmic actin or myosin VIIa through its DGR domain [349] and as an adaptor of Cul3-based E3 ligase that causes Nrf2 polyubiquitination and proteasomal degradation [30]. Two Keap1 proteins form a dimer via BTB domains and then bind Nrf2, through interactions between DGR domain of Keap1 and the Neh2 domain of Nrf2 [30, 350]. Importantly some cysteine residues in the cysteine-rich intervening region [351], especially cys 151, 273 and cys 288, are required for Nrf2 binding.
A very recent study from Palsamy and collaborators demonstrated that the unfolded protein response, stimulated with endoplasmic reticulum stressors and oxidants, caused loss of methylation of the KEAP1 promoter and up-regulated Keap1 expression thereby decreasing Nrf2 and Nrf2-target gene expression in human lens epithelial cells [352]. Interestingly they found that Keap1 promoter methylation was decreased in lenses in aging reaching the lowest level around 75 y. As oxidative stress triggers changes in DNA methylation pattern [353, 354] and accumulation of unfolded protein is increased in with age [355, 356], it is reasonable to hypothesize that Keap1 expression increases with age. Nonetheless, in a mouse model of accelerated aging (SAMP8), Tombe et al. observed a significant decrease in Nrf2 mRNA and its total and nuclear protein in the liver of 10 mo mice compared with age-matched normal SAMR1 mice, but did not observe an age-related change of Keap1 mRNA and protein levels [266]. Rahman et al. also did not observe an age-related change of Keap1 mRNA using 10–50 d flies [195].
Bach1
Bach proteins (Bach1 and Bach2) belong to B-Zip protein family. Through their BTB domain Bach proteins form dimers with small Maf proteins and bind to EpRE. Bach1 is universally expressed while Bach2 is mainly expressed in neural and monocytes [357]. Igarashi et al. first reported Bach1 as a transcription repressor evidenced by results that Bach1 could interact with MafK and bind to EpRE, but lacked a transactivation domain [358]. Bach2 was also identified as a repressor of EpRE activity [359]. Later the same group showed that Bach1 abrogated Nrf2/EpRE-mediated HO-1 induction [360]. Additional study from Suzuki and collaborators showed that the Nrf2/EpRE inducers cadmium could induce the nuclear export of Bach1 and Bach2, mediated by a C-terminal conserved domain with involvement of ERK1/2 signaling [361]. The competitive effect of Bach1 with Nrf2 was confirmed by later studies [362, 363], which demonstrated that Bach1 repressed tert-butylhydroquione-induced Nrf2/EpRE activation. Most Bach1 and Nrf2 locate in the cytoplasm under resting conditions, and both translocate into nucleus upon oxidative stress, with a delay of Bach1 nuclear translocation compared with Nrf2 [362, 363]. Using diamide, Ishikawa et al. showed that Bach1 activity was redox regulated [364] as modification of cysteine residues in Bach1’s DNA binding domain abrogated its DNA binding ability. Meng et al. showed that cysteine residues 557 and 574 were involved in Bach1 dissociation from EpRE complex of HO-1 confirming the redox-dependence of Bach1 activity [365]. In addition, Bach1 phosphorylation at tyrosine 486, which is induced by oxidants, is responsible for the nuclear export of Bach1 [366]. Interestingly, expression of Bach1 is regulated by Nrf2/EpRE signaling [367].
Due to the repressing effect of Bach1 on Nrf2/EpRE signaling, studies have examined the potential role of Bach1 in age-dependent decline of Nrf2 signaling. Bach1 forms a complex with p53, inhibiting p53 activity and thereby inhibiting senescence [368]. Using immunoprecipitation assays, Shenvi et al. showed that Bach1 binding to the functional EpRE in rat GCLC promoter was increased in cells from old mice, with a simultaneous decrease of Nrf2 binding [314]. We recently reported that the Bach1 protein levels in the lung, liver and cerebellum of middle-aged mice was significantly higher than those of young adults, and the increase of Bach1 was associated with a decline of Nrf2-targeted gene induction in response to nanoparticles [157]. As mentioned above, oxidative stress causes nuclear translocation of Bach1. Therefore we hypothesize that oxidative stress could cause an age-related nuclear accumulation of Bach1 and play a significant role in the age-related decline in Nrf2 signaling.
c-Myc
c-Myc is a proto-oncogene and also a transcription factor that regulates genes involved in cell growth, proliferation and apoptosis. Several lines of evidence suggest that c-Myc is a negative regulator of Nrf2 signaling. Our lab first demonstrated that silencing c-Myc increased expression of four Nrf2-regulated genes through both association with Nrf2-EpRE complexes in the nucleus and shortened Nrf2 half-life [369]. A very recent report from Yang and colleagues showed that Nrf2 signaling was down-regulated through a c-Myc-miR27a/b-PHB1 circuit. Lithocholic acid (LCA) induced c-Myc and reduced expression of Nrf2 and GCL in mice [370]. c-Myc promoted induction of microRNA 27a/b that targeted both PHB1 and Nrf2 to reduce their expression. Knockdown of c-Myc or miR27a/b attenuated LCA-mediated suppression of Nrf2 and GCL expression. Furthermore, c-Myc directly interacted with Nrf2 and lowered Nrf2 binding to EpRE [370]. Indeed, c-Myc was negatively associated with expression of Nrf2-target genes in various tissues of mice [157, 371]. Consistently, Burdo et al. showed that c-Myc phosphorylation reduced nuclear Nrf2 in rat neurons and down-regulated GCLC expression while inhibition of c-Myc phosphorylation reversed such changes [372]. In contrast, Nagy reported that c-Myc overexpression induced p62 and Nrf2 signaling in Drosophila [373] and in primary murine cells [374]. In all, c-Myc is an important participant, probably a negative regulator, of the regulatory network of Nrf2/EpRE signaling.
c-Myc mRNA was increased significantly in the liver of rats with aging (6, 12, 18, 24 mo) [375]. Semsei et al. determined the steady-state mRNA levels of c-Myc in various normal tissues throughout the life span of the C57BL/6J male mouse strain [376]. c-Myc expression was highest in prenatal and newborn ages and decreased to its lowest level at about 6 mo. With further increase of age, a progressive increase of c-Myc mRNA was found in brain, liver, skin, and small intestine. However, c-Myc mRNA level was not changed in the kidney, spleen and heart with aging [376]. A recent study from our lab also found c-Myc protein level was significantly higher in the lung, liver, and cerebellum of middle aged (21 mo) in comparison with young mice (6 mo) [157]. An earlier study showed that there was a retarded rate of c-Myc mRNA degradation in T-cells from older than young human subjects [377]. In contrast, Novikov et al. showed that c-Myc and c-Fos expression level was not changed in rat livers with aging [378]. Taken together, the available data suggest age-dependent change of c-Myc expression varies with species and tissues, and its expression is increased in some tissues. Recent data from our lab suggest that age-related increase of c-Myc is associated with the decline of Nrf2 signaling and induction of its target genes [157]. Obviously more studies are needed to elucidate the expression pattern of c-Myc in various tissues from different models, and also the potential role of c-Myc in age-dependent decline of Nrf2 signaling.
MicroRNAs in the regulation of Nrf2 signaling
MicroRNA (miRNA) are small non-coding RNA sequences containing about 22 nucleotides. The miRNA bind to the 3′ UTR of their target mRNA through complimentary base pairing, causing the degradation (high complementary) or blocking the translation (lower levels of complementarity) of the target mRNA. Therefore one gene may be targeted by many miRNA. MiRNA play key roles in regulating gene expression post-transcriptionally. MiRNA themselves are regulated at the transcriptional level through vartious transcription factors. For example, both Nrf2 and NF-κB regulate miRNA expression [379].
Accumulating evidence suggests that many miRNA may regulate Nrf2/EpRE activity through targeting the expression of proteins involved in Nrf2/EpRE pathway. Some miRNA regulate the Nrf2/EpRE pathway through directly targeting Nrf2. For example, miR-101-2 (Kim, Lee et al. 2014), miR-93 [380], miR-28 [381], miR-507, miR-634, miR-450a, miR-129-5p (Yamamoto, Inoue et al. 2014), and miR153 [382] can target Nrf2 mRNA and down regulate Nrf2 protein expression. In addition, many Nrf2 regulatory proteins and partners are also regulated by miRNA, and these miRNA could regulate Nrf2/EpRE signaling. Bach1, a negative regulator of Nrf2/EpRE activity, has been shown to be target of miR-196b [383], miR-155 [384], let-7b miRNA, let-7c miRNA, miR98 [385], and miR-122 [386]. Another negative regulator of Nrf2, c-Myc, was also regulated by miRNA, including miR-520d-3p [387], miRNA-135b [388], miR-34b/c [389], miR-744 [390], miR-184 [391], microRNA-135a [392], miR-145 [393] [394], miR-126 [395], miR-449c [396], miR-196b [397], miR-34a [398], miR-33b [399], miR-98 [400], miR-let-7 [400] [401] and miR-185-3p [402]. The Nrf2 partner, c-Jun is also targeted by miRNA miR-125b [403] and miR-155 [404]. Keap1, the Nrf2 inhibitor, is the target of miRNA miR-200a [405, 406] and miR-141 [407] [408]. With more studies, it is expected that additional miRNAs will be found to be involved in regulating Nrf2/EpRE signaling, by targeting Nrf2 or its associated proteins [409].
Some studies have examined the effect of aging on miRNA expression [410]. Regarding age-dependent changes of miRNA that target Nrf2 signaling, less information is available. Li and colleagues studied the expression level of miR-34a and miR-93 and its target gene Nrf2 in livers of rats and found that both miRNAs were increased significantly with aging (18, 24, 28 mo vs 4 mo), with a corresponding decrease of Nrf2 protein [411]. An increased expression of miR-34a was also observed in the retina and RPE of old mice (24, 28, 32 mo vs 4 mo) [412]. Csiszar et al recently reported that miR-144 was elevated in the cerebromicrovascular endothelial cells (CMVECs) from aged rats (24 mo vs 3 mo), and the level of Nrf2, its target gene, was decreased [413]. As more miRNA involved in Nrf2 regulation are identified, their roles in the impaired Nrf2 function with age should be determined. It is unclear if tissue or species differences exist in the age-dependent change of these miRNAs, and how these changes may contribute to the impaired Nrf2 function with aging. As one miRNA can target multiple genes, and one gene can be targeted by multiple miRNAs, an extremely complex situation is likely to represent a significant challenge to determining the interrelationships of miRNAs, Nrf2/EpRE and aging.
Conclusions and perspective
Advancing age is accompanied by an increase in oxidant production from various sources and a simultaneous dysfunction of the antioxidant defenses, leading to accumulated oxidative damage to proteins, nucleotides and lipids in aging cells. With advancing age, particularly as organisms become frail, susceptibility to oxidants and other toxicants increases [163, 175, 414]. In addition, redox signaling, which plays a key role in the adaptive response to oxidative stimuli, changes during aging [38]. Here we reviewed the age-related variation of several antioxidant enzymes, Nrf2/EpRE function, and some components of the Nrf2/EpRE regulatory system (Figure 1). Controversy remains about the direction of change in antioxidant enzymes especially Nrf2-regulated antioxidant genes, by aging itself. It is unclear if the controversy over the baseline changes with age is due to differences in the species, tissues, or cells actually studied, or to differences in aging status.
Figure 1.
Age-related change Nrf2 regulatory system. Green arrow shows positive and red arrow shows negative effect on target to which the arrow points
In contrast, accumulating evidence points to an age-related decline in the ability to respond to oxidative stress with activation of Nrf2/EpRE signaling and expression of its target antioxidant genes. Nonetheless, the complexity of the regulatory mechanisms for each antioxidant gene makes it difficult to clarify divergent findings. For example, HO-1 expression is regulated by multiple transcription factors including Nrf2/EpRE, NF-κB, and HIF-1α signaling. Although Nrf2/EpRE function declines with aging, both NF-κB signaling [415] and HIF-1α signaling are activated [184], which would lead to increased HO-1 expression in the old. Whether or not there is a tissue specific difference in the Nrf2/EpRE function represents another challenge for future study.
In reality, there are several big questions remaining to be answered. What mechanisms underlie the loss in response to oxidative stress of the Nrf2/EpRE signaling in old animals? To what degree can this decline in signaling competence be reversed? How does this loss in adaptability contribute to the senescent phenotype? Finally, how many of these effects of aging, largely studied, so far, only in animals, will also be important components of the aging phenotype in humans?
Table II.
Age-dependent change of GCL
| Marker | Change | Cell or tissue | Specie | Age | Reference |
|---|---|---|---|---|---|
| GCLC and GCLM | Decreased | Various | Fisher 344 rat, both m f | [160] | |
| GCLC and GCLM | Decreased, but resveratrol induced | Kidney | Wistar Rat, | 21 mo vs. 2 mo, 12 mo | [155] |
| GCLC and GCLM | Basal GCLC decreased, basal GCLM unchanged, GCLC but not GCLM was induced by hind-lime unloading | Soleus muscle | F344 rat | 26 mo vs. 13 mo | [135] |
| GCLC and GCLM | Decreased in activity, mRNA and protein | Liver, kidney, lung, RBC | F344 rat | [153] | |
| GCLC | Decreased | Carotid arteries, VSMC | Rhesus macaques | 20 y vs. 10 y | [137] |
| GCLC and GCLM | Decreased, lipoic acid induced | Liver | F344 rat | 24–28 mo vs. 2–5 mo | [147] |
| GCLM | Basal higher, impaired induction to sodium iodate, partially restored by KEAP1 knockout | Retinal pigment epithelium | Mice | 15 mo vs. 2 mo | [158] |
| GCLC | Basal decreased, induction declined in response to H2O2 and glucose | Aorta | Rat | 24 mo vs. 3 mo | [159] |
| GCLM and GCLC | GCLM decreased, GCLC unchanged | Brain | Rat | 17 mo vs. 4 mo | [161] |
Table IV.
age-dependent change of NQO-1
| Marker | Change | Cell or tissue | Specie | Age | Reference |
|---|---|---|---|---|---|
| NQO-1 | Decreased, restored by EGCG | Astrocytes | Mice | [190] | |
| NQO-1 | Basal increased, induction to nPM declined | Lung, liver, and cerebellum | Mice | 21 mo vs. 6 mo | [157] |
| NQO-1 | Basal decreased, induction declined in response to H2O2 and glucose | Aorta | Rat | 24 mo vs. 3 mo | [159] |
| NQO-1 | Not induced by toluene, but induced in hippocampus | Frontal cortex and cerebellum | Norway rat | 24 mo vs. 4,12 mo | [191] |
| NQO-1 | Basal not changed, induction by H2O2 blunted | Carotid artery, VSMC | Rhesus macaque | 20y vs. 10y | [137] |
| NQO-1 | Increased | Liver | Mice | 24 mo vs. 3 mo | [133] |
| NQO-1 | Increased, sulforaphane induced | Spleen lymphocytes | Mice | 19–21 mo vs. 2–4 mo | [156] |
| NQO-1 | Basal higher, impaired induction to sodium iodate, partially restored by KEAP1 knockout | Retinal pigment epithelium | Mice | 15 mo vs. 2 mo | [158] |
Highlights.
Induction of many antioxidant genes by oxidative stress declines with age
Nrf2 activation is impaired in aging with a loss of the electrophilic response
The Nrf2 positive regulators, PI3K, P62, CBP, and BRCA1, decrease with age
The Nrf2 negative regulators, Keap1, Bach1, and c-Myc, increase with age
MicroRNAs regulate Nrf2 signaling and may be involved in aging
Acknowledgments
This research was supported by United States NIH Grants R01-ES023864 and R01-ES003598.
Abbreviations
- BRCA1
Breast cancer susceptibility gene 1
- CBP
CREB binding protein
- EpRE
electrophile response element
- G6PDH
glucose-6-phosphate dehydrogenase
- GCL
glutamate cysteine ligase
- GCLC
glutamate cysteine ligase catalytic subunit
- GCLM
glutamate cysteine ligase modifier subunit
- GSH
glutathione
- NQO-1, NADPH
quinone oxidoreductase 1
- HO-1
heme oxygenase-1
- GPx
glutathione peroxidase
- GST
glutathione transferase
- Nrf2
nuclear factor erythroid 2 -related factor 2
- Prdx
peroxiredoxin
- p62
sequestosome-1
- SUMO
small ubiquitin-related modifier
- SOD
superoxide dismutase
- nPM
nanoparticle
- mo
months
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Butterfield DA, Howard BJ, LaFontaine MA. Brain oxidative stress in animal models of accelerated aging and the age-related neurodegenerative disorders, Alzheimer’s disease and Huntington’s disease. Curr Med Chem. 2001;8:815–828. doi: 10.2174/0929867013373048. [DOI] [PubMed] [Google Scholar]
- 2.Beal MF. Oxidatively modified proteins in aging and disease. Free radical biology & medicine. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 3.Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Jacob KD, Noren Hooten N, Trzeciak AR, Evans MK. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech Ageing Dev. 2013;134:139–157. doi: 10.1016/j.mad.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free radical biology & medicine. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slocum SL, Kensler TW. Nrf2: control of sensitivity to carcinogens. Arch Toxicol. 2011;85:273–284. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- 7.Toda N, Toda H. Coronary hemodynamic regulation by nitric oxide in experimental animals: recent advances. Eur J Pharmacol. 2011;667:41–49. doi: 10.1016/j.ejphar.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Takahashi S, Yamamoto M, Kawada T, Kudoh S, Sugawara I. Nrf2 is closely related to allergic airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol. 2010;137:234–241. doi: 10.1016/j.clim.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 10.Sykiotis GP, Bohmann D. Stress-activated cap‘n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura Y, Endo T. Survival responses to oxidative stress and aging. Geriatr Gerontol Int. 2010;10(Suppl 1):S1–9. doi: 10.1111/j.1447-0594.2010.00597.x. [DOI] [PubMed] [Google Scholar]
- 12.Davies KJ, Shringarpure R. Preferential degradation of oxidized proteins by the 20S proteasome may be inhibited in aging and in inflammatory neuromuscular diseases. Neurology. 2006;66:S93–96. doi: 10.1212/01.wnl.0000192308.43151.63. [DOI] [PubMed] [Google Scholar]
- 13.Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, Von Zglinicki T, Davies KJ. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14:1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- 14.Sitte N, Merker K, Von Zglinicki T, Davies KJ, Grune T. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part II--aging of nondividing cells. FASEB J. 2000;14:2503–2510. doi: 10.1096/fj.00-0210com. [DOI] [PubMed] [Google Scholar]
- 15.Sitte N, Merker K, Von Zglinicki T, Grune T, Davies KJ. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part I--effects of proliferative senescence. FASEB J. 2000;14:2495–2502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- 16.Shringarpure R, Davies KJ. Protein turnover by the proteasome in aging and disease. Free radical biology & medicine. 2002;32:1084–1089. doi: 10.1016/s0891-5849(02)00824-9. [DOI] [PubMed] [Google Scholar]
- 17.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 19.Bota DA, Van Remmen H, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS letters. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- 20.Ngo JK, Pomatto LC, Davies KJ. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013;1:258–264. doi: 10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Assar M, Angulo J, Rodriguez-Manas L. Oxidative stress and vascular inflammation in aging. Free radical biology & medicine. 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Volonte D, Liu Z, Musille PM, Stoppani E, Wakabayashi N, Di YP, Lisanti MP, Kensler TW, Galbiati F. Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol Biol Cell. 2013;24:1852–1862. doi: 10.1091/mbc.E12-09-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gounder SS, Kannan S, Devadoss D, Miller CJ, Whitehead KJ, Odelberg SJ, Firpo MA, Paine R, 3rd, Hoidal JR, Abel ED, Rajasekaran NS. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One. 2012;7:e45697. doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the betaglobin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friling RS, Bensimon A, Tichauer Y, Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci U S A. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Jaiswal AK. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J Biol Chem. 1992;267:15097–15104. [PubMed] [Google Scholar]
- 28.Sakai M, Okuda A, Muramatsu M. Multiple regulatory elements and phorbol 12-O-tetradecanoate 13-acetate responsiveness of the rat placental glutathione transferase gene. Proc Natl Acad Sci U S A. 1988;85:9456–9460. doi: 10.1073/pnas.85.24.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 30.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci U S A. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Forman HJ. Reexamination of the electrophile response element sequences and context reveals a lack of consensus in gene function. Biochim Biophys Acta. 2010;1799:496–501. doi: 10.1016/j.bbagrm.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen T, Rushmore TH, Pickett CB. Transcriptional regulation of a rat liver glutathione S-transferase Ya subunit gene. Analysis of the antioxidant response element and its activation by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate. J Biol Chem. 1994;269:13656–13662. [PubMed] [Google Scholar]
- 34.Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. Faseb J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Liu H, Dickinson DA, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. gamma-Glutamyl transpeptidase is induced by 4-hydroxynonenal via EpRE/Nrf2 signaling in rat epithelial type II cells. Free radical biology & medicine. 2006;40:1281–1292. doi: 10.1016/j.freeradbiomed.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 38.Sohal RS, Orr WC. The redox stress hypothesis of aging. Free radical biology & medicine. 2012;52:539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afanas’ev I. Signaling and Damaging Functions of Free Radicals in Aging-Free Radical Theory, Hormesis, and TOR. Aging Dis. 2010;1:75–88. [PMC free article] [PubMed] [Google Scholar]
- 40.Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vina J, Borras C, Abdelaziz KM, Garcia-Valles R, Gomez-Cabrera MC. The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid Redox Signal. 2013;19:779–787. doi: 10.1089/ars.2012.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stuart JA, Maddalena LA, Merilovich M, Robb EL. A midlife crisis for the mitochondrial free radical theory of aging. Longev Healthspan. 2014;3:4. doi: 10.1186/2046-2395-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Haeri M, Knox BE. Endoplasmic Reticulum Stress and Unfolded Protein Response Pathways: Potential for Treating Age-related Retinal Degeneration. J Ophthalmic Vis Res. 2012;7:45–59. [PMC free article] [PubMed] [Google Scholar]
- 45.Sohal RS, Orr WC. Relationship between antioxidants, prooxidants, and the aging process. Ann N Y Acad Sci. 1992;663:74–84. doi: 10.1111/j.1749-6632.1992.tb38651.x. [DOI] [PubMed] [Google Scholar]
- 46.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free radical biology & medicine. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 47.Sastre J, Pallardo FV, Vina J. The role of mitochondrial oxidative stress in aging. Free radical biology & medicine. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Court N, Forman HJ. Submicromolar concentrations of 4-hydroxynonenal induce glutamate cysteine ligase expression in HBE1 cells. Redox Rep. 2007;12:101–106. doi: 10.1179/135100007X162266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasegawa G, Fukui M, Hosoda H, Asano M, Harusato I, Tanaka M, Shiraishi E, Senmaru T, Sakabe K, Yamasaki M, Kitawaki J, Fujinami A, Ohta M, Obayashi H, Nakamura N. Telmisartan, an angiotensin II type 1 receptor blocker, prevents the development of diabetes in male Spontaneously Diabetic Torii rats. European journal of pharmacology. 2009;605:164–169. doi: 10.1016/j.ejphar.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS letters. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 51.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 53.Miller CJ, Gounder SS, Kannan S, Goutam K, Muthusamy VR, Firpo MA, Symons JD, Paine R, 3rd, Hoidal JR, Rajasekaran NS. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim Biophys Acta. 2012;1822:1038–1050. doi: 10.1016/j.bbadis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi S, Izawa Y, Suzuki N. Astroglial pentose phosphate pathway rates in response to high-glucose environments. ASN Neuro. 2012;4 doi: 10.1042/AN20120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 56.Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67:546–554. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- 57.Yao TP, Oh SP, Fuchs M, Zhou ND, Ch’ng LE, Newsome D, Bronson RT, Li E, Livingston DM, Eckner R. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93:361–372. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 58.Bae SH, Woo HA, Sung SH, Lee HE, Lee SK, Kil IS, Rhee SG. Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia. Antioxid Redox Signal. 2009;11:937–948. doi: 10.1089/ars.2008.2325. [DOI] [PubMed] [Google Scholar]
- 59.Soriano FX, Leveille F, Papadia S, Higgins LG, Varley J, Baxter P, Hayes JD, Hardingham GE. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- 61.Sakurai A, Nishimoto M, Himeno S, Imura N, Tsujimoto M, Kunimoto M, Hara S. Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: role of NF-E2-related factor-2. J Cell Physiol. 2005;203:529–537. doi: 10.1002/jcp.20246. [DOI] [PubMed] [Google Scholar]
- 62.Da Costa LA, Badawi A, El-Sohemy A. Nutrigenetics and modulation of oxidative stress. Ann Nutr Metab. 2012;60(Suppl 3):27–36. doi: 10.1159/000337311. [DOI] [PubMed] [Google Scholar]
- 63.Lu CY, Lee HC, Fahn HJ, Wei YH. Oxidative damage elicited by imbalance of free radical scavenging enzymes is associated with large-scale mtDNA deletions in aging human skin. Mutat Res. 1999;423:11–21. doi: 10.1016/s0027-5107(98)00220-6. [DOI] [PubMed] [Google Scholar]
- 64.Allen RG, Keogh BP, Gerhard GS, Pignolo R, Horton J, Cristofalo VJ. Expression and regulation of superoxide dismutase activity in human skin fibroblasts from donors of different ages. J Cell Physiol. 1995;165:576–587. doi: 10.1002/jcp.1041650316. [DOI] [PubMed] [Google Scholar]
- 65.Gautam N, Das S, Mahapatra SK, Chakraborty SP, Kundu PK, Roy S. Age associated oxidative damage in lymphocytes. Oxid Med Cell Longev. 2010;3:275–282. doi: 10.4161/oxim.3.4.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pansarasa O, Bertorelli L, Vecchiet J, Felzani G, Marzatico F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free radical biology & medicine. 1999;27:617–622. doi: 10.1016/s0891-5849(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 67.Tokunaga K, Kanno K, Ochi M, Nishimiya T, Shishino K, Murase M, Makino H, Tokui S. Lipid peroxide and antioxidants in the elderly. Rinsho Byori. 1998;46:783–789. [PubMed] [Google Scholar]
- 68.Guemouri L, Artur Y, Herbeth B, Jeandel C, Cuny G, Siest G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin Chem. 1991;37:1932–1937. [PubMed] [Google Scholar]
- 69.Andersen HR, Nielsen JB, Nielsen F, Grandjean P. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43:562–568. [PubMed] [Google Scholar]
- 70.Ji LL, Dillon D, Wu E. Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. Am J Physiol. 1990;258:R918–923. doi: 10.1152/ajpregu.1990.258.4.R918. [DOI] [PubMed] [Google Scholar]
- 71.Aydin AF, Kucukgergin C, Ozdemirler-Erata G, Kocak-Toker N, Uysal M. The effect of carnosine treatment on prooxidant-antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology. 2010;11:103–109. doi: 10.1007/s10522-009-9232-4. [DOI] [PubMed] [Google Scholar]
- 72.Vanella A, Geremia E, D’Urso G, Tiriolo P, Di Silvestro I, Grimaldi R, Pinturo R. Superoxide dismutase activities in aging rat brain. Gerontology. 1982;28:108–113. doi: 10.1159/000212519. [DOI] [PubMed] [Google Scholar]
- 73.Samarghandian S, Azimi-Nezhad M, Samini F. Preventive Effect of Safranal against Oxidative Damage in Aged Male Rat Brain. Exp Anim. 2015;64:7. doi: 10.1538/expanim.14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendoza-Nunez VM, Ruiz-Ramos M, Sanchez-Rodriguez MA, Retana-Ugalde R, Munoz-Sanchez JL. Aging-related oxidative stress in healthy humans. Tohoku J Exp Med. 2007;213:261–268. doi: 10.1620/tjem.213.261. [DOI] [PubMed] [Google Scholar]
- 75.Kasapoglu M, Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001;36:209–220. doi: 10.1016/s0531-5565(00)00198-4. [DOI] [PubMed] [Google Scholar]
- 76.Niwa Y, Iizawa O, Ishimoto K, Akamatsu H, Kanoh T. Age-dependent basal level and induction capacity of copper-zinc and manganese superoxide dismutase and other scavenging enzyme activities in leukocytes from young and elderly adults. Am J Pathol. 1993;143:312–320. [PMC free article] [PubMed] [Google Scholar]
- 77.Junqueira VB, Barros SB, Chan SS, Rodrigues L, Giavarotti L, Abud RL, Deucher GP. Aging and oxidative stress. Mol Aspects Med. 2004;25:5–16. doi: 10.1016/j.mam.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 78.Marzani B, Felzani G, Bellomo RG, Vecchiet J, Marzatico F. Human muscle aging: ROS-mediated alterations in rectus abdominis and vastus lateralis muscles. Exp Gerontol. 2005;40:959–965. doi: 10.1016/j.exger.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Vyskocilova E, Szotakova B, Skalova L, Bartikova H, Hlavacova J, Bousova I. Age-related changes in hepatic activity and expression of detoxification enzymes in male rats. Biomed Res Int. 2013;2013:408573. doi: 10.1155/2013/408573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodriguez-Capote K, Cespedes E, Arencibia R, Gonzalez-Hoyuela M. Indicators of oxidative stress in aging rat brain. The effect of nerve growth factor. Rev Neurol. 1998;27:494–500. [PubMed] [Google Scholar]
- 81.Mecocci P, Polidori MC, Troiano L, Cherubini A, Cecchetti R, Pini G, Straatman M, Monti D, Stahl W, Sies H, Franceschi C, Senin U. Plasma antioxidants and longevity: a study on healthy centenarians. Free radical biology & medicine. 2000;28:1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 82.Forman HJ, Kennedy JA. Role of superoxide radical in mitochondrial dehydrogenase reactions. Biochem Biophys Res Commun. 1974;60:1044–1050. doi: 10.1016/0006-291x(74)90418-5. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan-Gunn MJ, Lewandowski PA. Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr. 2013;13:104. doi: 10.1186/1471-2318-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sudheesh NP, Ajith TA, Ramnath V, Janardhanan KK. Therapeutic potential of Ganoderma lucidum (Fr.) P. Karst. against the declined antioxidant status in the mitochondria of post-mitotic tissues of aged mice. Clin Nutr. 2010;29:406–412. doi: 10.1016/j.clnu.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Landis G, Shen J, Tower J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging (Albany NY) 2012;4:768–789. doi: 10.18632/aging.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chandwaney R, Leichtweis S, Leeuwenburgh C, Ji LL. Oxidative stress and mitochondrial function in skeletal muscle: Effects of aging and exercise training. Age (Omaha) 1998;21:109–117. doi: 10.1007/s11357-998-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zuo Z, Lei H, Wang X, Wang Y, Sonntag W, Sun Z. Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. Age (Dordr) 2011;33:261–274. doi: 10.1007/s11357-010-9176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pechenino AS, Brown TR. Superoxide dismutase in the prostate lobes of aging Brown Norway rats. Prostate. 2006;66:522–535. doi: 10.1002/pros.20364. [DOI] [PubMed] [Google Scholar]
- 89.Gomes P, Simao S, Silva E, Pinto V, Amaral JS, Afonso J, Serrao MP, Pinho MJ, Soares-da-Silva P. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid Med Cell Longev. 2009;2:138–145. doi: 10.4161/oxim.2.3.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He Y, Tombran-Tink J. Mitochondrial decay and impairment of antioxidant defenses in aging RPE cells. Adv Exp Med Biol. 2010;664:165–183. doi: 10.1007/978-1-4419-1399-9_20. [DOI] [PubMed] [Google Scholar]
- 91.Starr ME, Ueda J, Yamamoto S, Evers BM, Saito H. The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free radical biology & medicine. 2011;50:371–380. doi: 10.1016/j.freeradbiomed.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moningka NC, Sindler AL, Muller-Delp JM, Baylis C. Twelve weeks of treadmill exercise does not alter age-dependent chronic kidney disease in the Fisher 344 male rat. J Physiol. 2011;589:6129–6138. doi: 10.1113/jphysiol.2011.214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fano G, Mecocci P, Vecchiet J, Belia S, Fulle S, Polidori MC, Felzani G, Senin U, Vecchiet L, Beal MF. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22:345–351. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- 94.Akcetin Z, Erdemli G, Bromme HJ. Experimental study showing a diminished cytosolic antioxidative capacity in kidneys of aged rats. Urol Int. 2000;64:70–73. doi: 10.1159/000030494. [DOI] [PubMed] [Google Scholar]
- 95.Helmy MM. Potential hepato-protective effect of alpha-tocopherol or simvastatin in aged rats. Pharmacol Rep. 2012;64:698–705. doi: 10.1016/s1734-1140(12)70864-2. [DOI] [PubMed] [Google Scholar]
- 96.Capel F, Buffiere C, Patureau Mirand P, Mosoni L. Differential variation of mitochondrial H2O2 release during aging in oxidative and glycolytic muscles in rats. Mech Ageing Dev. 2004;125:367–373. doi: 10.1016/j.mad.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Doria E, Buonocore D, Focarelli A, Marzatico F. Relationship between human aging muscle and oxidative system pathway. Oxid Med Cell Longev. 2012;2012:830257. doi: 10.1155/2012/830257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coling D, Chen S, Chi LH, Jamesdaniel S, Henderson D. Age-related changes in antioxidant enzymes related to hydrogen peroxide metabolism in rat inner ear. Neurosci Lett. 2009;464:22–25. doi: 10.1016/j.neulet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 100.Wingler K, Brigelius-Flohe R. Gastrointestinal glutathione peroxidase. Biofactors. 1999;10:245–249. doi: 10.1002/biof.5520100223. [DOI] [PubMed] [Google Scholar]
- 101.Conrad M, Schneider M, Seiler A, Bornkamm GW. Physiological role of phospholipid hydroperoxide glutathione peroxidase in mammals. Biol Chem. 2007;388:1019–1025. doi: 10.1515/BC.2007.130. [DOI] [PubMed] [Google Scholar]
- 102.Espinoza SE, Guo H, Fedarko N, DeZern A, Fried LP, Xue QL, Leng S, Beamer B, Walston JD. Glutathione peroxidase enzyme activity in aging. J Gerontol A Biol Sci Med Sci. 2008;63:505–509. doi: 10.1093/gerona/63.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inal ME, Kanbak G, Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta. 2001;305:75–80. doi: 10.1016/s0009-8981(00)00422-8. [DOI] [PubMed] [Google Scholar]
- 104.Rodriguez-Martinez MA, Alonso MJ, Redondo J, Salaices M, Marin J. Role of lipid peroxidation and the glutathione-dependent antioxidant system in the impairment of endothelium-dependent relaxations with age. Br J Pharmacol. 1998;123:113–121. doi: 10.1038/sj.bjp.0701595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rikans LE, Hornbrook KR. Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta. 1997;1362:116–127. doi: 10.1016/s0925-4439(97)00067-7. [DOI] [PubMed] [Google Scholar]
- 106.Kim HG, Hong SM, Kim SJ, Park HJ, Jung HI, Lee YY, Moon JS, Lim HW, Park EH, Lim CJ. Age-related changes in the activity of antioxidant and redox enzymes in rats. Mol Cells. 2003;16:278–284. [PubMed] [Google Scholar]
- 107.de Haan JB, Newman JD, Kola I. Cu/Zn superoxide dismutase mRNA and enzyme activity, and susceptibility to lipid peroxidation, increases with aging in murine brains. Brain Res Mol Brain Res. 1992;13:179–187. doi: 10.1016/0169-328x(92)90025-7. [DOI] [PubMed] [Google Scholar]
- 108.Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD, Muller-Delp JM. Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol (1985) 2013;114:681–693. doi: 10.1152/japplphysiol.00341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He T, Joyner MJ, Katusic ZS. Aging decreases expression and activity of glutathione peroxidase-1 in human endothelial progenitor cells. Microvasc Res. 2009;78:447–452. doi: 10.1016/j.mvr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weir CP, Robaire B. Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. J Androl. 2007;28:229–240. doi: 10.2164/jandrol.106.001362. [DOI] [PubMed] [Google Scholar]
- 111.Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod. 2011;84:775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ogawa T, Boylan SA, Oltjen SL, Hjelmeland LM. Changes in the spatial expression of genes with aging in the mouse RPE/choroid. Mol Vis. 2005;11:380–386. [PubMed] [Google Scholar]
- 113.Xu W, Li H, Wang R, Lei Z, Mao Y, Wang X, Zhang Y, Guo T, Song R, Zhang X, Jin L, Li Z, Irwin DM, Niu G, Tan H. Differential expression of genes associated with the progression of renal disease in the kidneys of liver-specific glucokinase gene knockout mice. Int J Mol Sci. 2013;14:6467–6486. doi: 10.3390/ijms14036467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mendoza-Nunez VM, Beristain-Perez A, Perez-Vera SP, Altamirano-Lozano MA. Age-related sex differences in glutathione peroxidase and oxidative DNA damage in a healthy Mexican population. J Womens Health (Larchmt) 2010;19:919–926. doi: 10.1089/jwh.2009.1684. [DOI] [PubMed] [Google Scholar]
- 115.Linard A, Macaire J, Christon R. Phospholipid hydroperoxide glutathione peroxidase activity and vitamin E level in the liver microsomal membrane: effects of age and dietary alpha-linolenic acid deficiency. J Nutr Biochem. 2001;12:481–491. doi: 10.1016/s0955-2863(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 116.Zhang LP, Maiorino M, Roveri A, Ursini F. Phospholipid hydroperoxide glutathione peroxidase: specific activity in tissues of rats of different age and comparison with other glutathione peroxidases. Biochim Biophys Acta. 1989;1006:140–143. doi: 10.1016/0005-2760(89)90336-6. [DOI] [PubMed] [Google Scholar]
- 117.Zhu H, Santo A, Li Y. The antioxidant enzyme peroxiredoxin and its protective role in neurological disorders. Exp Biol Med (Maywood) 2012;237:143–149. doi: 10.1258/ebm.2011.011152. [DOI] [PubMed] [Google Scholar]
- 118.Perkins A, Poole LB, Karplus PA. Tuning of peroxiredoxin catalysis for various physiological roles. Biochemistry. 2014;53:7693–7705. doi: 10.1021/bi5013222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Musicco C, Capelli V, Pesce V, Timperio AM, Calvani M, Mosconi L, Zolla L, Cantatore P, Gadaleta MN. Accumulation of overoxidized Peroxiredoxin III in aged rat liver mitochondria. Biochim Biophys Acta. 2009;1787:890–896. doi: 10.1016/j.bbabio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 120.Yoo KY, Park OK, Yu J, Yan B, Li H, Lee CH, Choi JH, Kim DW, Hwang IK, Won MH. Expression and changes of hyperoxidized peroxiredoxins in non-pyramidal and polymorphic cells in the gerbil hippocampus during normal aging. Cell Mol Neurobiol. 2009;29:413–421. doi: 10.1007/s10571-008-9333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Molin M, Yang J, Hanzen S, Toledano MB, Labarre J, Nystrom T. Life span extension and H(2)O(2) resistance elicited by caloric restriction require the peroxiredoxin Tsa1 in Saccharomyces cerevisiae. Mol Cell. 2011;43:823–833. doi: 10.1016/j.molcel.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 122.Wang W, Gou L, Xie G, Tong A, He F, Lu Z, Yao Y, Liu K, Li J, Tang M, Chen L, Yang J, Hu H, Wei YQ. Proteomic analysis of interstitial fluid in bone marrow identified that peroxiredoxin 2 regulates H(2)O(2) level of bone marrow during aging. J Proteome Res. 2010;9:3812–3819. doi: 10.1021/pr901180w. [DOI] [PubMed] [Google Scholar]
- 123.Ding J, Kopchick JJ. Plasma biomarkers of mouse aging. Age (Dordr) 2011;33:291–307. doi: 10.1007/s11357-010-9179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Picca A, Pesce V, Fracasso F, Joseph AM, Leeuwenburgh C, Lezza AM. Aging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liver. PLoS One. 2013;8:e74644. doi: 10.1371/journal.pone.0074644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu JL, Vallat JM, Pollard JD, Knoops B, Ouvrier R. Expression of the antioxidant enzyme peroxiredoxin 5 in the human peripheral nervous system. J Peripher Nerv Syst. 2006;11:318–324. doi: 10.1111/j.1529-8027.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- 126.Kubo E, Miyazawa T, Fatma N, Akagi Y, Singh DP. Development- and age-associated expression pattern of peroxiredoxin 6, and its regulation in murine ocular lens. Mech Ageing Dev. 2006;127:249–256. doi: 10.1016/j.mad.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 127.Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev. 2011;43:92–137. doi: 10.3109/03602532.2011.567391. [DOI] [PubMed] [Google Scholar]
- 128.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 129.Loguercio C, Taranto D, Vitale LM, Beneduce F, Del Vecchio Blanco C. Effect of liver cirrhosis and age on the glutathione concentration in the plasma, erythrocytes, and gastric mucosa of man. Free radical biology & medicine. 1996;20:483–488. doi: 10.1016/0891-5849(96)02057-6. [DOI] [PubMed] [Google Scholar]
- 130.Farahmand SK, Samini F, Samini M, Samarghandian S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology. 2013;14:63–71. doi: 10.1007/s10522-012-9409-0. [DOI] [PubMed] [Google Scholar]
- 131.van Lieshout EM, Peters WH. Age and gender dependent levels of glutathione and glutathione S-transferases in human lymphocytes. Carcinogenesis. 1998;19:1873–1875. doi: 10.1093/carcin/19.10.1873. [DOI] [PubMed] [Google Scholar]
- 132.Carrillo MC, Nokubo M, Kitani K, Satoh K, Sato K. Age-related alterations of enzyme activities and subunits of hepatic glutathione S-transferases in male and female Fischer-344 rats. Biochim Biophys Acta. 1991;1077:325–331. doi: 10.1016/0167-4838(91)90547-d. [DOI] [PubMed] [Google Scholar]
- 133.Fu ZD, Csanaky IL, Klaassen CD. Effects of aging on mRNA profiles for drug-metabolizing enzymes and transporters in livers of male and female mice. Drug Metab Dispos. 2012;40:1216–1225. doi: 10.1124/dmd.111.044461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ceballos-Picot I, Trivier JM, Nicole A, Sinet PM, Thevenin M. Age-correlated modifications of copper-zinc superoxide dismutase and glutathione-related enzyme activities in human erythrocytes. Clin Chem. 1992;38:66–70. [PubMed] [Google Scholar]
- 135.Chen CN, Brown-Borg HM, Rakoczy SG, Ferrington DA, Thompson LV. Aging impairs the expression of the catalytic subunit of glutamate cysteine ligase in soleus muscle under stress. J Gerontol A Biol Sci Med Sci. 2010;65:129–137. doi: 10.1093/gerona/glp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4:288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 137.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta EG, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. The journals of gerontology. Series A, Biological sciences and medical sciences. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Emir UE, Raatz S, McPherson S, Hodges JS, Torkelson C, Tawfik P, White T, Terpstra M. Noninvasive quantification of ascorbate and glutathione concentration in the elderly human brain. NMR Biomed. 2011;24:888–894. doi: 10.1002/nbm.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am J Clin Nutr. 2011;94:847–853. doi: 10.3945/ajcn.110.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lang CA, Naryshkin S, Schneider DL, Mills BJ, Lindeman RD. Low blood glutathione levels in healthy aging adults. J Lab Clin Med. 1992;120:720–725. [PubMed] [Google Scholar]
- 141.Toroser D, Sohal RS. Age-associated perturbations in glutathione synthesis in mouse liver. Biochem J. 2007;405:583–589. doi: 10.1042/BJ20061868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rebrin I, Sohal RS. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Deliv Rev. 2008;60:1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gould NS, Min E, Gauthier S, Chu HW, Martin R, Day BJ. Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung. Am J Respir Crit Care Med. 2010;182:1114–1122. doi: 10.1164/rccm.201003-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Toroser D, Sohal RS. Kinetic characteristics of native gamma-glutamylcysteine ligase in the aging housefly, Musca domestica L. Biochem Biophys Res Commun. 2005;326:586–593. doi: 10.1016/j.bbrc.2004.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang H, Liu H, Liu RM. Gender difference in glutathione metabolism during aging in mice. Exp Gerontol. 2003;38:507–517. doi: 10.1016/s0531-5565(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 147.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu RM, Kavanagh TJ, Forman HJ. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem Biophys. 2004;423:116–125. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 149.Shi MM, Kugelman A, Iwamoto T, Tian L, Forman HJ. Quinone-induced oxidative stress elevates glutathione and induces gamma-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J Biol Chem. 1994;269:26512–26517. [PubMed] [Google Scholar]
- 150.Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 151.Moinova HR, Mulcahy RT. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun. 1999;261:661–668. doi: 10.1006/bbrc.1999.1109. [DOI] [PubMed] [Google Scholar]
- 152.Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free radical biology & medicine. 2002;33:974. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 153.Liu R, Choi J. Age-associated decline in gamma-glutamylcysteine synthetase gene expression in rats. Free radical biology & medicine. 2000;28:566–574. doi: 10.1016/s0891-5849(99)00269-5. [DOI] [PubMed] [Google Scholar]
- 154.Liu RM. Down-regulation of gamma-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J Neurosci Res. 2002;68:344–351. doi: 10.1002/jnr.10217. [DOI] [PubMed] [Google Scholar]
- 155.Yuan J, Zhang Z, Li L, Song W. Resveratrol affects the expression of glutamate cysteine ligase in the kidneys of aged rats. Exp Ther Med. 2014;7:1762–1766. doi: 10.3892/etm.2014.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim HJ, Nel AE. The role of phase II antioxidant enzymes in protecting memory T cells from spontaneous apoptosis in young and old mice. J Immunol. 2005;175:2948–2959. doi: 10.4049/jimmunol.175.5.2948. [DOI] [PubMed] [Google Scholar]
- 157.Zhang H, Liu H, Davies KJ, Sioutas C, Finch CE, Morgan TE, Forman HJ. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free radical biology & medicine. 2012;52:2038–2046. doi: 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sachdeva MM, Cano M, Handa JT. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. American journal of physiology. Heart and circulatory physiology. 2011;301:H363–372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu H, Wang H, Shenvi S, Hagen TM, Liu RM. Glutathione metabolism during aging and in Alzheimer disease. Ann N Y Acad Sci. 2004;1019:346–349. doi: 10.1196/annals.1297.059. [DOI] [PubMed] [Google Scholar]
- 161.Zhu Y, Carvey PM, Ling Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006;1090:35–44. doi: 10.1016/j.brainres.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kaliman PA, Nikitchenko IV. Activity of key enzymes of heme metabolism and the content of several hemoproteins in the liver of rats of various ages. Ukr Biokhim Zh. 1989;61:75–78. [PubMed] [Google Scholar]
- 163.Harder Y, Amon M, Georgi M, Scheuer C, Schramm R, Rucker M, Pittet B, Erni D, Menger MD. Aging is associated with an increased susceptibility to ischaemic necrosis due to microvascular perfusion failure but not a reduction in ischaemic tolerance. Clin Sci (Lond) 2007;112:429–440. doi: 10.1042/CS20060187. [DOI] [PubMed] [Google Scholar]
- 164.Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J Neurotrauma. 2009;26:1557–1566. doi: 10.1089/neu.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tabuchi K, Hoshino T, Hirose Y, Hayashi K, Nishimura B, Nakayama M, Hara A. Age-related hearing loss and expression of antioxidant enzymes in BDF1 mice. Acta Otolaryngol. 2011;131:1020–1024. doi: 10.3109/00016489.2011.589406. [DOI] [PubMed] [Google Scholar]
- 166.Ito Y, Betsuyaku T, Moriyama C, Nasuhara Y, Nishimura M. Aging affects lipopolysaccharide-induced upregulation of heme oxygenase-1 in the lungs and alveolar macrophages. Biogerontology. 2009;10:173–180. doi: 10.1007/s10522-008-9164-4. [DOI] [PubMed] [Google Scholar]
- 167.Ewing JF, Maines MD. Regulation and expression of heme oxygenase enzymes in aged-rat brain: age related depression in HO-1 and HO-2 expression and altered stress-response. J Neural Transm. 2006;113:439–454. doi: 10.1007/s00702-005-0408-z. [DOI] [PubMed] [Google Scholar]
- 168.Hirose W, Ikematsu K, Tsuda R. Age-associated increases in heme oxygenase-1 and ferritin immunoreactivity in the autopsied brain. Leg Med (Tokyo) 2003;5(Suppl 1):S360–366. doi: 10.1016/s1344-6223(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 169.Reckelhoff JF, Kanji V, Racusen LC, Schmidt AM, Yan SD, Marrow J, Roberts LJ, 2nd, Salahudeen AK. Vitamin E ameliorates enhanced renal lipid peroxidation and accumulation of F2-isoprostanes in aging kidneys. Am J Physiol. 1998;274:R767–774. doi: 10.1152/ajpregu.1998.274.3.R767. [DOI] [PubMed] [Google Scholar]
- 170.Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89:687–699. doi: 10.1016/s0306-4522(98)00334-0. [DOI] [PubMed] [Google Scholar]
- 171.Barnes CJ, Cameron IL, Puleo-Scheppke B, Lee M. Age alters expression and inducibility of heme oxygenase isozymes in mice. Age (Omaha) 1998;21:123–128. doi: 10.1007/s11357-998-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bloomer SA, Zhang HJ, Brown KE, Kregel KC. Differential regulation of hepatic heme oxygenase-1 protein with aging and heat stress. J Gerontol A Biol Sci Med Sci. 2009;64:419–425. doi: 10.1093/gerona/gln056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nakanishi Y, Yasumoto K. Induction after administering paraquat of heme oxygenase-1 and heat shock protein 70 in the liver of senescence-accelerated mice. Biosci Biotechnol Biochem. 1997;61:1302–1306. doi: 10.1271/bbb.61.1302. [DOI] [PubMed] [Google Scholar]
- 174.Di Giulio C, Verratti V, Artese L, Petruccelli G, Walski M, Pokorski M. Aging and expression of heme oxygenase-1 and endothelin-1 in the rat carotid body after chronic hypoxia. J Physiol Pharmacol. 2009;60(Suppl 5):41–44. [PubMed] [Google Scholar]
- 175.Moore BA, Albers KM, Davis BM, Grandis JR, Togel S, Bauer AJ. Altered inflammatory gene expression underlies increased susceptibility to murine postoperative ileus with advancing age. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1650–1659. doi: 10.1152/ajpgi.00570.2006. [DOI] [PubMed] [Google Scholar]
- 176.Lima CF, Pereira-Wilson C, Rattan SI. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: relevance for anti-aging intervention. Mol Nutr Food Res. 2011;55:430–442. doi: 10.1002/mnfr.201000221. [DOI] [PubMed] [Google Scholar]
- 177.Nath KA, Grande JP, Farrugia G, Croatt AJ, Belcher JD, Hebbel RP, Vercellotti GM, Katusic ZS. Age sensitizes the kidney to heme protein-induced acute kidney injury. Am J Physiol Renal Physiol. 2013;304:F317–325. doi: 10.1152/ajprenal.00606.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Patriarca S, Furfaro AL, Cosso L, Pesce Maineri E, Balbis E, Domenicotti C, Nitti M, Cottalasso D, Marinari UM, Pronzato MA, Traverso N. Heme oxygenase 1 expression in rat liver during ageing and ethanol intoxication. Biogerontology. 2007;8:365–372. doi: 10.1007/s10522-006-9079-x. [DOI] [PubMed] [Google Scholar]
- 179.Abraham NG, Levere RD, Freedman ML. Effect of age on rat liver heme and drug metabolism. Exp Gerontol. 1985;20:277–284. doi: 10.1016/0531-5565(85)90053-1. [DOI] [PubMed] [Google Scholar]
- 180.Bitar MS, Shapiro BH. Aberration of heme and hemoprotein in aged female rats. Mech Ageing Dev. 1987;38:189–197. doi: 10.1016/0047-6374(87)90078-9. [DOI] [PubMed] [Google Scholar]
- 181.Iijima N, Tamada Y, Hayashi S, Tanaka M, Ishihara A, Hasegawa M, Ibata Y. Expanded expression of heme oxygenase-1 (HO-1) in the hypothalamic median eminence of aged as compared with young rats: an immunocytochemical study. Neurosci Lett. 1999;271:113–116. doi: 10.1016/s0304-3940(99)00543-1. [DOI] [PubMed] [Google Scholar]
- 182.Kim HJ, Jung KJ, Seo AY, Choi JS, Yu BP, Chung HY. Calorie restriction modulates redox-sensitive AP-1 during the aging process. J Am Aging Assoc. 2002;25:123–130. doi: 10.1007/s11357-002-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Colombrita C, Calabrese V, Stella AM, Mattei F, Alkon DL, Scapagnini G. Regional rat brain distribution of heme oxygenase-1 and manganese superoxide dismutase mRNA: relevance of redox homeostasis in the aging processes. Exp Biol Med (Maywood) 2003;228:517–524. doi: 10.1177/15353702-0322805-16. [DOI] [PubMed] [Google Scholar]
- 184.Kang MJ, Kim HJ, Kim HK, Lee JY, Kim DH, Jung KJ, Kim KW, Baik HS, Yoo MA, Yu BP, Chung HY. The effect of age and calorie restriction on HIF-1-responsive genes in aged liver. Biogerontology. 2005;6:27–37. doi: 10.1007/s10522-004-7381-z. [DOI] [PubMed] [Google Scholar]
- 185.Kireev RA, Tresguerres AC, Garcia C, Borras C, Ariznavarreta C, Vara E, Vina J, Tresguerres JA. Hormonal regulation of pro-inflammatory and lipid peroxidation processes in liver of old ovariectomized female rats. Biogerontology. 2010;11:229–243. doi: 10.1007/s10522-009-9242-2. [DOI] [PubMed] [Google Scholar]
- 186.Ferenbach DA, Nkejabega NC, McKay J, Choudhary AK, Vernon MA, Beesley MF, Clay S, Conway BC, Marson LP, Kluth DC, Hughes J. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int. 2011;79:966–976. doi: 10.1038/ki.2010.535. [DOI] [PubMed] [Google Scholar]
- 187.Thulluri S, Wu M, Blough ER, Manne ND, Litchfield AB, Wang B. Regulation of iron-related molecules in the rat hippocampus: sex- and age-associated differences. Ann Clin Lab Sci. 2012;42:145–151. [PubMed] [Google Scholar]
- 188.Tucsek Z, Gautam T, Sonntag WE, Toth P, Saito H, Salomao R, Szabo C, Csiszar A, Ungvari Z. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci. 2013;68:652–660. doi: 10.1093/gerona/gls232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gleixner AM, Pulugulla SH, Pant DB, Posimo JM, Crum TS, Leak RK. Impact of aging on heat shock protein expression in the substantia nigra and striatum of the female rat. Cell Tissue Res. 2014;357:43–54. doi: 10.1007/s00441-014-1852-6. [DOI] [PubMed] [Google Scholar]
- 190.Duan W, Zhang R, Guo Y, Jiang Y, Huang Y, Jiang H, Li C. Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. In Vitro Cell Dev Biol Anim. 2009;45:388–397. doi: 10.1007/s11626-009-9194-5. [DOI] [PubMed] [Google Scholar]
- 191.Kodavanti PR, Royland JE, Richards JE, Besas J, Macphail RC. Toluene effects on oxidative stress in brain regions of young-adult, middle-age, and senescent Brown Norway rats. Toxicol Appl Pharmacol. 2011;256:386–398. doi: 10.1016/j.taap.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 192.Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8:71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- 193.Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.George L, Lokhandwala MF, Asghar M. Exercise activates redox-sensitive transcription factors and restores renal D1 receptor function in old rats. Am J Physiol Renal Physiol. 2009;297:F1174–1180. doi: 10.1152/ajprenal.00397.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rahman MM, Sykiotis GP, Nishimura M, Bodmer R, Bohmann D. Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell. 2013;12:554–562. doi: 10.1111/acel.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 198.Kojima T, Dogru M, Higuchi A, Nagata T, Ibrahim OM, Inaba T, Tsubota K. The effect of Nrf2 knockout on ocular surface protection from acute tobacco smoke exposure: evidence from Nrf2 knockout mice. Am J Pathol. 2015;185:776–785. doi: 10.1016/j.ajpath.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 199.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Khor TO, Huang MT, Prawan A, Liu Y, Hao X, Yu S, Cheung WK, Chan JY, Reddy BS, Yang CS, Kong AN. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila) 2008;1:187–191. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cheung KL, Lee JH, Khor TO, Wu TY, Li GX, Chan J, Yang CS, Kong AN. Nrf2 knockout enhances intestinal tumorigenesis in Apc(min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation. Mol Carcinog. 2014;53:77–84. doi: 10.1002/mc.21950. [DOI] [PubMed] [Google Scholar]
- 202.Meakin PJ, Chowdhry S, Sharma RS, Ashford FB, Walsh SV, McCrimmon RJ, Dinkova-Kostova AT, Dillon JF, Hayes JD, Ashford ML. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol Cell Biol. 2014;34:3305–3320. doi: 10.1128/MCB.00677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hoshino T, Tabuchi K, Nishimura B, Tanaka S, Nakayama M, Ishii T, Warabi E, Yanagawa T, Shimizu R, Yamamoto M, Hara A. Protective role of Nrf2 in age-related hearing loss and gentamicin ototoxicity. Biochem Biophys Res Commun. 2011;415:94–98. doi: 10.1016/j.bbrc.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 204.Chen H, Jin S, Guo J, Kombairaju P, Biswal S, Zirkin BR. Knockout of the transcription factor Nrf2: Effects on testosterone production by aging mouse Leydig cells. Molecular and cellular endocrinology. 2015;409:113–120. doi: 10.1016/j.mce.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hirota A, Kawachi Y, Yamamoto M, Koga T, Hamada K, Otsuka F. Acceleration of UVB-induced photoageing in nrf2 gene-deficient mice. Exp Dermatol. 2011;20:664–668. doi: 10.1111/j.1600-0625.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 206.Taguchi K, Maher JM, Suzuki T, Kawatani Y, Motohashi H, Yamamoto M. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol Cell Biol. 2010;30:3016–3026. doi: 10.1128/MCB.01591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Grune T, Catalgol B, Licht A, Ermak G, Pickering AM, Ngo JK, Davies KJ. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free radical biology & medicine. 2011;51:1355–1364. doi: 10.1016/j.freeradbiomed.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Howden R. Nrf2 and cardiovascular defense. Oxid Med Cell Longev. 2013;2013:104308. doi: 10.1155/2013/104308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gan L, Johnson JA. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta. 2014;1842:1208–1218. doi: 10.1016/j.bbadis.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 210.Boutten A, Goven D, Artaud-Macari E, Bonay M. Protective role of Nrf2 in the lungs against oxidative airway diseases. Med Sci (Paris) 2011;27:966–972. doi: 10.1051/medsci/20112711012. [DOI] [PubMed] [Google Scholar]
- 211.Zhang DD. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13:1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 212.Deramaudt TB, Dill C, Bonay M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Med Mal Infect. 2013;43:100–107. doi: 10.1016/j.medmal.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 213.Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 214.Niture SK, Jain AK, Jaiswal AK. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J Cell Sci. 2009;122:4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 215.Zhang H, Forman HJ. Acrolein induces heme oxygenase-1 through PKC-delta and PI3K in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2008;38:483–490. doi: 10.1165/rcmb.2007-0260OC. [DOI] [PubMed] [Google Scholar]
- 216.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free radical biology & medicine. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 217.Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS letters. 2003;546:181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- 218.Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free radical biology & medicine. 2006;41:1079–1091. doi: 10.1016/j.freeradbiomed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 219.Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- 220.Ahmed Atia AA. The Nrf2-Keap1 Signalling Pathway: Mechanisms of ARE transcription regulation in antioxidant cellular defence. Int J PharmTech Res. 2014;6:14. [Google Scholar]
- 221.Zipper LM, Mulcahy RT. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci. 2003;73:124–134. doi: 10.1093/toxsci/kfg083. [DOI] [PubMed] [Google Scholar]
- 222.Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 223.Zhang H, Liu H, Iles KE, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. 4-Hydroxynonenal induces rat gamma-glutamyl transpeptidase through mitogen-activated protein kinase-mediated electrophile response element/nuclear factor erythroid 2-related factor 2 signaling. Am J Respir Cell Mol Biol. 2006;34:174–181. doi: 10.1165/rcmb.2005-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang X, Chen HL, Liu JZ, Liao N, Yu WH, Zhang XD, Zhang T, Li WL, Hai CX. Protective effect of oleanolic acid against beta cell dysfunction and mitochondrial apoptosis: crucial role of ERK-NRF2 signaling pathway. J Biol Regul Homeost Agents. 2013;27:55–67. [PubMed] [Google Scholar]
- 225.Cong ZX, Wang HD, Wang JW, Zhou Y, Pan H, Zhang DD, Zhu L. ERK and PI3K signaling cascades induce Nrf2 activation and regulate cell viability partly through Nrf2 in human glioblastoma cells. Oncol Rep. 2013;30:715–722. doi: 10.3892/or.2013.2485. [DOI] [PubMed] [Google Scholar]
- 226.Wang J, Zhang L, Zhang Y, Luo M, Wu Q, Yu L, Chu H. Transcriptional upregulation centra of HO-1 by EGB via the MAPKs/Nrf2 pathway in mouse C2C12 myoblasts. Toxicol In Vitro. 2015;29:380–388. doi: 10.1016/j.tiv.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 227.Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 228.Kachadourian R, Pugazhenthi S, Velmurugan K, Backos DS, Franklin CC, McCord JM, Day BJ. 2′,5′-Dihydroxychalcone-induced glutathione is mediated by oxidative stress and kinase signaling pathways. Free radical biology & medicine. 2011;51:1146–1154. doi: 10.1016/j.freeradbiomed.2011.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Deng X, Rui W, Zhang F, Ding W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol Toxicol. 2013;29:143–157. doi: 10.1007/s10565-013-9242-5. [DOI] [PubMed] [Google Scholar]
- 230.Zhang H, Forman HJ. Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol Ind Health. 2009;25:269–278. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang C, Blough E, Arvapalli R, Dai X, Triest WE, Leidy JW, Masannat Y, Wu M. Acetaminophen attenuates glomerulosclerosis in obese Zucker rats via ROS/p38MAPK signaling pathways. Free radical biology & medicine. 2015 doi: 10.1016/j.freeradbiomed.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 232.Deng C, Sun Z, Tong G, Yi W, Ma L, Zhao B, Cheng L, Zhang J, Cao F, Yi D. alpha-Lipoic acid reduces infarct size and preserves cardiac function in rat myocardial ischemia/reperfusion injury through activation of PI3K/Akt/Nrf2 pathway. PLoS One. 2013;8:e58371. doi: 10.1371/journal.pone.0058371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang J, Hu X, Xie J, Xu W, Jiang H. Beta-1-Adrenergic Receptors Mediate Nrf2-HO-1-HMGB1 Axis Regulation to Attenuate Hypoxia/Reoxygenation-Induced Cardiomyocytes Injury in Vitro. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;35:767–777. doi: 10.1159/000369736. [DOI] [PubMed] [Google Scholar]
- 234.Aggarwal BB, Deb L, Prasad S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules. 2014;20:185–205. doi: 10.3390/molecules20010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Park EJ, Park SW, Kim HJ, Kwak JH, Lee DU, Chang KC. Dehydrocostuslactone inhibits LPS-induced inflammation by p38MAPK-dependent induction of hemeoxygenase-1 in vitro and improves survival of mice in CLP-induced sepsis in vivo. Int Immunopharmacol. 2014;22:332–340. doi: 10.1016/j.intimp.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 236.Zhao J, Feng L, Liu Y, Jiang W, Wu P, Jiang J, Zhang Y, Zhou X. Effect of dietary isoleucine on the immunity, antioxidant status, tight junctions and microflora in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian) Fish Shellfish Immunol. 2014;41:663–673. doi: 10.1016/j.fsi.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 237.Kocanova S, Buytaert E, Matroule JY, Piette J, Golab J, de Witte P, Agostinis P. Induction of heme-oxygenase 1 requires the p38MAPK and PI3K pathways and suppresses apoptotic cell death following hypericin-mediated photodynamic therapy. Apoptosis. 2007;12:731–741. doi: 10.1007/s10495-006-0016-x. [DOI] [PubMed] [Google Scholar]
- 238.Zhao W, Ma G, Chen X. Lipopolysaccharide induced LOX-1 expression via TLR4/MyD88/ROS activated p38MAPK-NF-kappaB pathway. Vascul Pharmacol. 2014;63:162–172. doi: 10.1016/j.vph.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 239.Keum YS, Yu S, Chang PP, Yuan X, Kim JH, Xu C, Han J, Agarwal A, Kong AN. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 240.Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Molecular cancer therapeutics. 2006;5:1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- 241.Yuan X, Xu C, Pan Z, Keum YS, Kim JH, Shen G, Yu S, Oo KT, Ma J, Kong AN. Butylated hydroxyanisole regulates ARE-mediated gene expression via Nrf2 coupled with ERK and JNK signaling pathway in HepG2 cells. Mol Carcinog. 2006;45:841–850. doi: 10.1002/mc.20234. [DOI] [PubMed] [Google Scholar]
- 242.Qin S, Deng F, Wu W, Jiang L, Yamashiro T, Yano S, Hou DX. Baicalein modulates Nrf2/Keap1 system in both Keap1-dependent and Keap1-independent mechanisms. Archives of biochemistry and biophysics. 2014;559:53–61. doi: 10.1016/j.abb.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 243.Vari R, D’Archivio M, Filesi C, Carotenuto S, Scazzocchio B, Santangelo C, Giovannini C, Masella R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J Nutr Biochem. 2011;22:409–417. doi: 10.1016/j.jnutbio.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 244.Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free radical biology & medicine. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jain AK, Jaiswal AK. Phosphorylation of tyrosine 568 controls nuclear export of Nrf2. J Biol Chem. 2006;281:12132–12142. doi: 10.1074/jbc.M511198200. [DOI] [PubMed] [Google Scholar]
- 246.Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 247.Kaspar JW, Jaiswal AK. Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression. FASEB J. 2011;25:1076–1087. doi: 10.1096/fj.10-171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bitar MS, Al-Mulla F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;301:E1119–1129. doi: 10.1152/ajpendo.00047.2011. [DOI] [PubMed] [Google Scholar]
- 249.Koo JH, Lee WH, Lee CG, Kim SG. Fyn inhibition by cycloalkane-fused 1,2-dithiole-3-thiones enhances antioxidant capacity and protects mitochondria from oxidative injury. Mol Pharmacol. 2012;82:27–36. doi: 10.1124/mol.111.077149. [DOI] [PubMed] [Google Scholar]
- 250.Stachel I, Geismann C, Aden K, Deisinger F, Rosenstiel P, Schreiber S, Sebens S, Arlt A, Schafer H. Modulation of nuclear factor E2-related factor-2 (Nrf2) activation by the stress response gene immediate early response-3 (IER3) in colonic epithelial cells: a novel mechanism of cellular adaption to inflammatory stress. J Biol Chem. 2014;289:1917–1929. doi: 10.1074/jbc.M113.490920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhou X, Zhao L, Mao J, Huang J, Chen J. Antioxidant Effects of Hydrogen Sulfide on Left Ventricular Remodeling in Smoking Rats Are Mediated via PI3K/Akt-Dependent Activation of Nrf2. Toxicol Sci. 2014 doi: 10.1093/toxsci/kfu272. [DOI] [PubMed] [Google Scholar]
- 252.Li M, Li C, Parkhouse WS. Age-related differences in the des IGF-I-mediated activation of Akt-1 and p70 S6K in mouse skeletal muscle. Mech Ageing Dev. 2003;124:771–778. doi: 10.1016/s0047-6374(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 253.Shay KP, Hagen TM. Age-associated impairment of Akt phosphorylation in primary rat hepatocytes is remediated by alpha-lipoic acid through PI3 kinase, PTEN, and PP2A. Biogerontology. 2009;10:443–456. doi: 10.1007/s10522-008-9187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Martineau LC, Chadan SG, Parkhouse WS. Age-associated alterations in cardiac and skeletal muscle glucose transporters, insulin and IGF-1 receptors, and PI3-kinase protein contents in the C57BL/6 mouse. Mech Ageing Dev. 1999;106:217–232. doi: 10.1016/s0047-6374(98)00106-7. [DOI] [PubMed] [Google Scholar]
- 255.Centurione L, Antonucci A, Miscia S, Grilli A, Rapino M, Grifone G, Di Giacomo V, Di Giulio C, Falconi M, Cataldi A. Age-related death-survival balance in myocardium: an immunohistochemical and biochemical study. Mech Ageing Dev. 2002;123:341–350. doi: 10.1016/s0047-6374(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 256.Takahashi H, Okamura D, Starr ME, Saito H, Evers BM. Age-dependent reduction of the PI3K regulatory subunit p85alpha suppresses pancreatic acinar cell proliferation. Aging Cell. 2012;11:305–314. doi: 10.1111/j.1474-9726.2011.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jiang T, Yin F, Yao J, Brinton RD, Cadenas E. Lipoic acid restores age-associated impairment of brain energy metabolism through the modulation of Akt/JNK signaling and PGC1alpha transcriptional pathway. Aging Cell. 2013;12:1021–1031. doi: 10.1111/acel.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y, Fu L. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav Brain Res. 2014;264:82–90. doi: 10.1016/j.bbr.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 259.Verschoor CP, Johnstone J, Loeb M, Bramson JL, Bowdish DM. Anti-pneumococcal deficits of monocyte-derived macrophages from the advanced-age, frail elderly and related impairments in PI3K-AKT signaling. Hum Immunol. 2014;75:1192–1196. doi: 10.1016/j.humimm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 260.Noren Hooten N, Abdelmohsen K, Gorospe M, Ejiogu N, Zonderman AB, Evans MK. microRNA expression patterns reveal differential expression of target genes with age. PLoS One. 2010;5:e10724. doi: 10.1371/journal.pone.0010724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bektas A, Zhang Y, Lehmann E, Wood WH, 3rd, Becker KG, Madara K, Ferrucci L, Sen R. Age-associated changes in basal NF-kappaB function in human CD4+ T lymphocytes via dysregulation of PI3 kinase. Aging (Albany NY) 2014;6:957–974. doi: 10.18632/aging.100705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Arias EB, Gosselin LE, Cartee GD. Exercise training eliminates age-related differences in skeletal muscle insulin receptor and IRS-1 abundance in rats. J Gerontol A Biol Sci Med Sci. 2001;56:B449–455. doi: 10.1093/gerona/56.10.b449. [DOI] [PubMed] [Google Scholar]
- 263.Castello L, Maina M, Testa G, Cavallini G, Biasi F, Donati A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto E. Alternate-day fasting reverses the age-associated hypertrophy phenotype in rat heart by influencing the ERK and PI3K signaling pathways. Mech Ageing Dev. 2011;132:305–314. doi: 10.1016/j.mad.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 264.Fallah MP, Chelvarajan RL, Garvy BA, Bondada S. Role of phosphoinositide 3-kinase-Akt signaling pathway in the age-related cytokine dysregulation in splenic macrophages stimulated via TLR-2 or TLR-4 receptors. Mech Ageing Dev. 2011;132:274–286. doi: 10.1016/j.mad.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Majumdar AP, Du J. Phosphatidylinositol 3-kinase/Akt signaling stimulates colonic mucosal cell survival during aging. Am J Physiol Gastrointest Liver Physiol. 2006;290:G49–55. doi: 10.1152/ajpgi.00106.2005. [DOI] [PubMed] [Google Scholar]
- 266.Tomobe K, Shinozuka T, Kuroiwa M, Nomura Y. Age-related changes of Nrf2 and phosphorylated GSK-3beta in a mouse model of accelerated aging (SAMP8) Arch Gerontol Geriatr. 2012;54:e1–7. doi: 10.1016/j.archger.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 267.Ramani K, Tomasi ML, Yang H, Ko K, Lu SC. Mechanism and significance of changes in glutamate-cysteine ligase expression during hepatic fibrogenesis. J Biol Chem. 2012;287:36341–36355. doi: 10.1074/jbc.M112.370775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tomasi ML, Ryoo M, Yang H, Iglesias Ara A, Ko KS, Lu SC. Molecular mechanisms of lipopolysaccharide-mediated inhibition of glutathione synthesis in mice. Free radical biology & medicine. 2014;68:148–158. doi: 10.1016/j.freeradbiomed.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Malloy MT, McIntosh DJ, Walters TS, Flores A, Goodwin JS, Arinze IJ. Trafficking of the transcription factor Nrf2 to promyelocytic leukemia-nuclear bodies: implications for degradation of NRF2 in the nucleus. J Biol Chem. 2013;288:14569–14583. doi: 10.1074/jbc.M112.437392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bossis G, Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell. 2006;21:349–357. doi: 10.1016/j.molcel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 271.Xu Z, Lam LS, Lam LH, Chau SF, Ng TB, Au SW. Molecular basis of the redox regulation of SUMO proteases: a protective mechanism of intermolecular disulfide linkage against irreversible sulfhydryl oxidation. FASEB J. 2008;22:127–137. doi: 10.1096/fj.06-7871com. [DOI] [PubMed] [Google Scholar]
- 272.Sang J, Yang K, Sun Y, Han Y, Cang H, Chen Y, Shi G, Wang K, Zhou J, Wang X, Yi J. SUMO2 and SUMO3 transcription is differentially regulated by oxidative stress in an Sp1-dependent manner. Biochem J. 2011;435:489–498. doi: 10.1042/BJ20101474. [DOI] [PubMed] [Google Scholar]
- 273.Li T, Santockyte R, Shen RF, Tekle E, Wang G, Yang DC, Chock PB. Expression of SUMO-2/3 induced senescence through p53- and pRB-mediated pathways. J Biol Chem. 2006;281:36221–36227. doi: 10.1074/jbc.M608236200. [DOI] [PubMed] [Google Scholar]
- 274.Shishido T, Woo CH, Ding B, McClain C, Molina CA, Yan C, Yang J, Abe J. Effects of MEK5/ERK5 association on small ubiquitin-related modification of ERK5: implications for diabetic ventricular dysfunction after myocardial infarction. Circ Res. 2008;102:1416–1425. doi: 10.1161/CIRCRESAHA.107.168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ivanschitz L, De The H, Le Bras M. PML, SUMOylation, and Senescence. Front Oncol. 2013;3:171. doi: 10.3389/fonc.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhang L, Li F, Dimayuga E, Craddock J, Keller JN. Effects of aging and dietary restriction on ubiquitination, sumoylation, and the proteasome in the spleen. FEBS letters. 2007;581:5543–5547. doi: 10.1016/j.febslet.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Li F, Zhang L, Craddock J, Bruce-Keller AJ, Dasuri K, Nguyen A, Keller JN. Aging and dietary restriction effects on ubiquitination, sumoylation, and the proteasome in the heart. Mech Ageing Dev. 2008;129:515–521. doi: 10.1016/j.mad.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yang QG, Wang F, Zhang Q, Xu WR, Chen YP, Chen GH. Correlation of increased hippocampal Sumo3 with spatial learning ability in old C57BL/6 mice. Neurosci Lett. 2012;518:75–79. doi: 10.1016/j.neulet.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 279.Sapir A, Tsur A, Koorman T, Ching K, Mishra P, Bardenheier A, Podolsky L, Bening-Abu-Shach U, Boxem M, Chou TF, Broday L, Sternberg PW. Controlled sumoylation of the mevalonate pathway enzyme HMGS-1 regulates metabolism during aging. Proc Natl Acad Sci U S A. 2014;111:E3880–3889. doi: 10.1073/pnas.1414748111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gorospe M, Wang X, Holbrook NJ. Functional role of p21 during the cellular response to stress. Gene Expr. 1999;7:377–385. [PMC free article] [PubMed] [Google Scholar]
- 281.Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Buitrago-Molina LE, Marhenke S, Longerich T, Sharma AD, Boukouris AE, Geffers R, Guigas B, Manns MP, Vogel A. The degree of liver injury determines the role of p21 in liver regeneration and hepatocarcinogenesis in mice. Hepatology. 2013;58:1143–1152. doi: 10.1002/hep.26412. [DOI] [PubMed] [Google Scholar]
- 284.Anantharaju A, Feller A, Chedid A. Aging Liver. A review. Gerontology. 2002;48:343–353. doi: 10.1159/000065506. [DOI] [PubMed] [Google Scholar]
- 285.Weinberg WC, Denning MF. P21Waf1 control of epithelial cell cycle and cell fate. Crit Rev Oral Biol Med. 2002;13:453–464. doi: 10.1177/154411130201300603. [DOI] [PubMed] [Google Scholar]
- 286.Famulski KS, Halloran PF. Molecular events in kidney ageing. Curr Opin Nephrol Hypertens. 2005;14:243–248. doi: 10.1097/01.mnh.0000165890.60254.4e. [DOI] [PubMed] [Google Scholar]
- 287.Herbig U, Sedivy JM. Regulation of growth arrest in senescence: telomere damage is not the end of the story. Mech Ageing Dev. 2006;127:16–24. doi: 10.1016/j.mad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 288.Deng Y, Chan SS, Chang S. Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer. 2008;8:450–458. doi: 10.1038/nrc2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Enomoto K, Mimura T, Harris DL, Joyce NC. Age differences in cyclin-dependent kinase inhibitor expression and rb hyperphosphorylation in human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2006;47:4330–4340. doi: 10.1167/iovs.05-1581. [DOI] [PubMed] [Google Scholar]
- 290.Simon K, Mukundan A, Dewundara S, Van Remmen H, Dombkowski AA, Cabelof DC. Transcriptional profiling of the age-related response to genotoxic stress points to differential DNA damage response with age. Mech Ageing Dev. 2009;130:637–647. doi: 10.1016/j.mad.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Song Z, Wang Y, Xie L, Zang X, Yin H. Expression of senescence-related genes in human corneal endothelial cells. Mol Vis. 2008;14:161–170. [PMC free article] [PubMed] [Google Scholar]
- 292.Yi YW, Kang HJ, Bae I. BRCA1 and Oxidative Stress. Cancers (Basel) 2014;6:771–795. doi: 10.3390/cancers6020771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bae I, Fan S, Meng Q, Rih JK, Kim HJ, Kang HJ, Xu J, Goldberg ID, Jaiswal AK, Rosen EM. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Res. 2004;64:7893–7909. doi: 10.1158/0008-5472.CAN-04-1119. [DOI] [PubMed] [Google Scholar]
- 294.Gorrini C, Baniasadi PS, Harris IS, Silvester J, Inoue S, Snow B, Joshi PA, Wakeham A, Molyneux SD, Martin B, Bouwman P, Cescon DW, Elia AJ, Winterton-Perks Z, Cruickshank J, Brenner D, Tseng A, Musgrave M, Berman HK, Khokha R, Jonkers J, Mak TW, Gauthier ML. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J Exp Med. 2013;210:1529–1544. doi: 10.1084/jem.20121337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Somasundaram K, Zhang H, Zeng YX, Houvras Y, Peng Y, Wu GS, Licht JD, Weber BL, El-Deiry WS. Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature. 1997;389:187–190. doi: 10.1038/38291. [DOI] [PubMed] [Google Scholar]
- 296.Ouchi T, Monteiro AN, August A, Aaronson SA, Hanafusa H. BRCA1 regulates p53-dependent gene expression. Proc Natl Acad Sci U S A. 1998;95:2302–2306. doi: 10.1073/pnas.95.5.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Pao GM, Janknecht R, Ruffner H, Hunter T, Verma IM. CBP/p300 interact with and function as transcriptional coactivators of BRCA1. Proc Natl Acad Sci U S A. 2000;97:1020–1025. doi: 10.1073/pnas.97.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wang Q, Zhang H, Kajino K, Greene MI. BRCA1 binds c-Myc and inhibits its transcriptional and transforming activity in cells. Oncogene. 1998;17:1939–1948. doi: 10.1038/sj.onc.1202403. [DOI] [PubMed] [Google Scholar]
- 299.Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Govindaraj V, Keralapura Basavaraju R, Rao AJ. Changes in the expression of DNA double strand break repair genes in primordial follicles from immature and aged rats. Reprod Biomed Online. 2014 doi: 10.1016/j.rbmo.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 301.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5:172ra121. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Evans TA, Raina AK, Delacourte A, Aprelikova O, Lee HG, Zhu X, Perry G, Smith MA. BRCA1 may modulate neuronal cell cycle re-entry in Alzheimer disease. Int J Med Sci. 2007;4:140–145. doi: 10.7150/ijms.4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shen G, Hebbar V, Nair S, Xu C, Li W, Lin W, Keum YS, Han J, Gallo MA, Kong AN. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem. 2004;279:23052–23060. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- 304.Zhu M, Fahl WE. Functional characterization of transcription regulators that interact with the electrophile response element. Biochem Biophys Res Commun. 2001;289:212–219. doi: 10.1006/bbrc.2001.5944. [DOI] [PubMed] [Google Scholar]
- 305.Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 306.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 307.Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 308.Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Li Q, Xiao H, Isobe K. Histone acetyltransferase activities of cAMP-regulated enhancer-binding protein and p300 in tissues of fetal, young, and old mice. J Gerontol A Biol Sci Med Sci. 2002;57:B93–98. doi: 10.1093/gerona/57.3.b93. [DOI] [PubMed] [Google Scholar]
- 310.Matsumoto A. Age-related changes in nuclear receptor coactivator immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. Brain Res. 2002;943:202–205. doi: 10.1016/s0006-8993(02)02622-7. [DOI] [PubMed] [Google Scholar]
- 311.Chung YH, Kim EJ, Shin CM, Joo KM, Kim MJ, Woo HW, Cha CI. Age-related changes in CREB binding protein immunoreactivity in the cerebral cortex and hippocampus of rats. Brain Res. 2002;956:312–318. doi: 10.1016/s0006-8993(02)03562-x. [DOI] [PubMed] [Google Scholar]
- 312.Tomas Pereira I, Coletta CE, Perez EV, Kim DH, Gallagher M, Goldberg IG, Rapp PR. CREB-binding protein levels in the rat hippocampus fail to predict chronological or cognitive aging. Neurobiol Aging. 2013;34:832–844. doi: 10.1016/j.neurobiolaging.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Radak Z, Bori Z, Koltai E, Fatouros IG, Jamurtas AZ, Douroudos, Terzis G, Nikolaidis MG, Chatzinikolaou A, Sovatzidis A, Kumagai S, Naito H, Boldogh I. Age-dependent changes in 8-oxoguanine-DNA glycosylase activity are modulated by adaptive responses to physical exercise in human skeletal muscle. Free radical biology & medicine. 2011;51:417–423. doi: 10.1016/j.freeradbiomed.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Shenvi SV, Smith E, Hagen TM. Identification of age-specific Nrf2 binding to a novel antioxidant response element locus in the Gclc promoter: a compensatory means for the loss of glutathione synthetic capacity in the aging rat liver? Aging Cell. 2012;11:297–304. doi: 10.1111/j.1474-9726.2011.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ziady AG, Sokolow A, Shank S, Corey D, Myers R, Plafker S, Kelley TJ. Interaction with CREB binding protein modulates the activities of Nrf2 and NF-kappaB in cystic fibrosis airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1221–1231. doi: 10.1152/ajplung.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 317.Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci. 2012;37:230–236. doi: 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Liu Y, Kern JT, Walker JR, Johnson JA, Schultz PG, Luesch H. A genomic screen for activators of the antioxidant response element. Proc Natl Acad Sci U S A. 2007;104:5205–5210. doi: 10.1073/pnas.0700898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E, Zhang DD. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Mol Cell Biol. 2013;33:2436–2446. doi: 10.1128/MCB.01748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Teshiba R, Tajiri T, Sumitomo K, Masumoto K, Taguchi T, Yamamoto K. Identification of a KEAP1 germline mutation in a family with multinodular goitre. PLoS One. 2013;8:e65141. doi: 10.1371/journal.pone.0065141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kwon J, Han E, Bui CB, Shin W, Lee J, Lee S, Choi YB, Lee AH, Lee KH, Park C, Obin MS, Park SK, Seo YJ, Oh GT, Lee HW, Shin J. Assurance of mitochondrial integrity and mammalian longevity by the p62-Keap1-Nrf2-Nqo1 cascade. EMBO Rep. 2012;13:150–156. doi: 10.1038/embor.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, Lee MS, Tanaka K, Komatsu M. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol. 2011;106:1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 326.Soontornniyomkij V, Risbrough VB, Young JW, Soontornniyomkij B, Jeste DV, Achim CL. Increased hippocampal accumulation of autophagosomes predicts short-term recognition memory impairment in aged mice. Age (Dordr) 2012;34:305–316. doi: 10.1007/s11357-011-9234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cui J, Bai XY, Shi S, Cui S, Hong Q, Cai G, Chen X. Age-related changes in the function of autophagy in rat kidneys. Age (Dordr) 2012;34:329–339. doi: 10.1007/s11357-011-9237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Liu Y, Shi S, Gu Z, Du Y, Liu M, Yan S, Gao J, Li J, Shao Y, Zhong W, Chen X, Li C. Impaired autophagic function in rat islets with aging. Age (Dordr) 2013;35:1531–1544. doi: 10.1007/s11357-012-9456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Chen K, Yang YH, Jiang SD, Jiang LS. Decreased activity of osteocyte autophagy with aging may contribute to the bone loss in senile population. Histochem Cell Biol. 2014;142:285–295. doi: 10.1007/s00418-014-1194-1. [DOI] [PubMed] [Google Scholar]
- 330.Cui J, Shi S, Sun X, Cai G, Cui S, Hong Q, Chen X, Bai XY. Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS One. 2013;8:e69720. doi: 10.1371/journal.pone.0069720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Luo L, Lu AM, Wang Y, Hong A, Chen Y, Hu J, Li X, Qin ZH. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp Gerontol. 2013;48:427–436. doi: 10.1016/j.exger.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 332.Bartlett BJ, Isakson P, Lewerenz J, Sanchez H, Kotzebue RW, Cumming RC, Harris GL, Nezis IP, Schubert DR, Simonsen A, Finley KD. p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy. 2011;7:572–583. doi: 10.4161/auto.7.6.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Myeku N, Figueiredo-Pereira ME. Dynamics of the degradation of ubiquitinated proteins by proteasomes and autophagy: association with sequestosome 1/p62. J Biol Chem. 2011;286:22426–22440. doi: 10.1074/jbc.M110.149252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.McMullen CA, Ferry AL, Gamboa JL, Andrade FH, Dupont-Versteegden EE. Age-related changes of cell death pathways in rat extraocular muscle. Exp Gerontol. 2009;44:420–425. doi: 10.1016/j.exger.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 337.Hayes JD, McMahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001;174:103–113. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- 338.He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 339.Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol. 2008;38:541–550. doi: 10.1165/rcmb.2007-0221OC. [DOI] [PubMed] [Google Scholar]
- 340.Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol. 2010;30:1007–1013. doi: 10.1161/ATVBAHA.110.204354. [DOI] [PubMed] [Google Scholar]
- 341.Miyamoto N, Izumi H, Miyamoto R, Bin H, Kondo H, Tawara A, Sasaguri Y, Kohno K. Transcriptional regulation of activating transcription factor 4 under oxidative stress in retinal pigment epithelial ARPE-19/HPV-16 cells. Invest Ophthalmol Vis Sci. 2011;52:1226–1234. doi: 10.1167/iovs.10-5775. [DOI] [PubMed] [Google Scholar]
- 342.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.van Huizen R, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2alpha signaling. J Biol Chem. 2003;278:15558–15564. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- 344.Li W, Miller RA. Elevated ATF4 Function in Fibroblasts and Liver of Slow-Aging Mutant Mice. J Gerontol A Biol Sci Med Sci. 2015;70:263–272. doi: 10.1093/gerona/glu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Hussain SG, Ramaiah KV. Reduced eIF2alpha phosphorylation and increased proapoptotic proteins in aging. Biochem Biophys Res Commun. 2007;355:365–370. doi: 10.1016/j.bbrc.2007.01.156. [DOI] [PubMed] [Google Scholar]
- 346.Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol (1985) 2011;111:135–142. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci U S A. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 351.Sorci G, Faivre B. Inflammation and oxidative stress in vertebrate host-parasite systems. Philos Trans R Soc Lond B Biol Sci. 2009;364:71–83. doi: 10.1098/rstb.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Palsamy P, Bidasee KR, Ayaki M, Augusteyn RC, Chan JY, Shinohara T. Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age-related cataracts. Free radical biology & medicine. 2014;72:134–148. doi: 10.1016/j.freeradbiomed.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Turk PW, Laayoun A, Smith SS, Weitzman SA. DNA adduct 8-hydroxyl-2′-deoxyguanosine (8-hydroxyguanine) affects function of human DNA methyltransferase. Carcinogenesis. 1995;16:1253–1255. doi: 10.1093/carcin/16.5.1253. [DOI] [PubMed] [Google Scholar]
- 354.Weitzman SA, Turk PW, Milkowski DH, Kozlowski K. Free radical adducts induce alterations in DNA cytosine methylation. Proc Natl Acad Sci U S A. 1994;91:1261–1264. doi: 10.1073/pnas.91.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Naidoo N. ER and aging-Protein folding and the ER stress response. Ageing Res Rev. 2009;8:150–159. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 356.Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 357.Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Igarashi K, Hoshino H, Muto A, Suwabe N, Nishikawa S, Nakauchi H, Yamamoto M. Multivalent DNA binding complex generated by small Maf and Bach1 as a possible biochemical basis for beta-globin locus control region complex. J Biol Chem. 1998;273:11783–11790. doi: 10.1074/jbc.273.19.11783. [DOI] [PubMed] [Google Scholar]
- 359.Hoshino H, Kobayashi A, Yoshida M, Kudo N, Oyake T, Motohashi H, Hayashi N, Yamamoto M, Igarashi K. Oxidative stress abolishes leptomycin B-sensitive nuclear export of transcription repressor Bach2 that counteracts activation of Maf recognition element. J Biol Chem. 2000;275:15370–15376. doi: 10.1074/jbc.275.20.15370. [DOI] [PubMed] [Google Scholar]
- 360.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Suzuki H, Tashiro S, Sun J, Doi H, Satomi S, Igarashi K. Cadmium induces nuclear export of Bach1, a transcriptional repressor of heme oxygenase-1 gene. J Biol Chem. 2003;278:49246–49253. doi: 10.1074/jbc.M306764200. [DOI] [PubMed] [Google Scholar]
- 362.Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20:2651–2653. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 363.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 364.Ishikawa M, Numazawa S, Yoshida T. Redox regulation of the transcriptional repressor Bach1. Free radical biology & medicine. 2005;38:1344–1352. doi: 10.1016/j.freeradbiomed.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 365.Meng D, Wang X, Chang Q, Hitron A, Zhang Z, Xu M, Chen G, Luo J, Jiang B, Fang J, Shi X. Arsenic promotes angiogenesis in vitro via a heme oxygenase-1-dependent mechanism. Toxicol Appl Pharmacol. 2010;244:291–299. doi: 10.1016/j.taap.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 366.Kaspar JW, Jaiswal AK. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J Biol Chem. 2010;285:153–162. doi: 10.1074/jbc.M109.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Jyrkkanen HK, Kuosmanen S, Heinaniemi M, Laitinen H, Kansanen E, Mella-Aho E, Leinonen H, Yla-Herttuala S, Levonen AL. Novel insights into the regulation of antioxidant-response-element- mediated gene expression by electrophiles: induction of the transcriptional repressor BACH1 by Nrf2. Biochem J. 2011;440:167–174. doi: 10.1042/BJ20110526. [DOI] [PubMed] [Google Scholar]
- 368.Dohi Y, Ikura T, Hoshikawa Y, Katoh Y, Ota K, Nakanome A, Muto A, Omura S, Ohta T, Ito A, Yoshida M, Noda T, Igarashi K. Bach1 inhibits oxidative stress-induced cellular senescence by impeding p53 function on chromatin. Nat Struct Mol Biol. 2008;15:1246–1254. doi: 10.1038/nsmb.1516. [DOI] [PubMed] [Google Scholar]
- 369.Levy S, Forman HJ. c-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB Life. 2010;62:237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Yang H, Li TW, Zhou Y, Peng H, Liu T, Zandi E, Martinez-Chantar ML, Mato JM, Lu SC. Activation of a novel c-Myc-miR27-prohibitin 1 circuitry in cholestatic liver injury inhibits glutathione synthesis in mice. Antioxid Redox Signal. 2015;22:259–274. doi: 10.1089/ars.2014.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Jian B, Yang S, Chaudry IH, Raju R. Resveratrol restores sirtuin 1 (SIRT1) activity and pyruvate dehydrogenase kinase 1 (PDK1) expression after hemorrhagic injury in a rat model. Mol Med. 2014;20:10–16. doi: 10.2119/molmed.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Burdo J, Schubert D, Maher P. Glutathione production is regulated via distinct pathways in stressed and non-stressed cortical neurons. Brain Res. 2008;1189:12–22. doi: 10.1016/j.brainres.2007.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Nagy P, Varga A, Pircs K, Hegedus K, Juhasz G. Myc-driven overgrowth requires unfolded protein response-mediated induction of autophagy and antioxidant responses in Drosophila melanogaster. PLoS Genet. 2013;9:e1003664. doi: 10.1371/journal.pgen.1003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Matocha MF, Cosgrove JW, Atack JR, Rapoport SI. Selective elevation of c-myc transcript levels in the liver of the aging Fischer-344 rat. Biochem Biophys Res Commun. 1987;147:1–7. doi: 10.1016/s0006-291x(87)80078-5. [DOI] [PubMed] [Google Scholar]
- 376.Semsei I, Ma SY, Cutler RG. Tissue and age specific expression of the myc proto-oncogene family throughout the life span of the C57BL/6J mouse strain. Oncogene. 1989;4:465–471. [PubMed] [Google Scholar]
- 377.Deguchi Y, Negoro S, Hara H, Nishio S, Kishimoto S. Age-related changes of proliferative response, kinetics of expression of protooncogenes after the mitogenic stimulation and methylation level of the protooncogene in purified human lymphocyte subsets. Mech Ageing Dev. 1988;44:153–168. doi: 10.1016/0047-6374(88)90087-5. [DOI] [PubMed] [Google Scholar]
- 378.Novikov LB, Balanskii RM, Anisimov VN, Khanson KP. Proto-oncogene expression in the liver of male rats of different age. Biull Eksp Biol Med. 1991;111:521–522. [PubMed] [Google Scholar]
- 379.Shah NM, Rushworth SA, Murray MY, Bowles KM, MacEwan DJ. Understanding the role of NRF2-regulated miRNAs in human malignancies. Oncotarget. 2013;4:1130–1142. doi: 10.18632/oncotarget.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Singh B, Ronghe AM, Chatterjee A, Bhat NK, Bhat HK. MicroRNA-93 regulates NRF2 expression and is associated with breast carcinogenesis. Carcinogenesis. 2013;34:1165–1172. doi: 10.1093/carcin/bgt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Yang M, Yao Y, Eades G, Zhang Y, Zhou Q. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast cancer research and treatment. 2011;129:983–991. doi: 10.1007/s10549-011-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Narasimhan M, Riar AK, Rathinam ML, Vedpathak D, Henderson G, Mahimainathan L. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol Lett. 2014;228:179–191. doi: 10.1016/j.toxlet.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Hou W, Tian Q, Zheng J, Bonkovsky HL. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatology. 2010;51:1494–1504. doi: 10.1002/hep.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Du ZM, Hu LF, Wang HY, Yan LX, Zeng YX, Shao JY, Ernberg I. Upregulation of MiR-155 in nasopharyngeal carcinoma is partly driven by LMP1 and LMP2A and downregulates a negative prognostic marker JMJD1A. PLoS One. 2011;6:e19137. doi: 10.1371/journal.pone.0019137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Hou W, Tian Q, Steuerwald NM, Schrum LW, Bonkovsky HL. The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes. Biochim Biophys Acta. 2012;1819:1113–1122. doi: 10.1016/j.bbagrm.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Shan Y, Zheng J, Lambrecht RW, Bonkovsky HL. Reciprocal effects of micro-RNA-122 on expression of heme oxygenase-1 and hepatitis C virus genes in human hepatocytes. Gastroenterology. 2007;133:1166–1174. doi: 10.1053/j.gastro.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Li R, Yuan W, Mei W, Yang K, Chen Z. MicroRNA 520d-3p inhibits gastric cancer cell proliferation, migration, and invasion by downregulating EphA2 expression. Molecular and cellular biochemistry. 2014;396:295–305. doi: 10.1007/s11010-014-2164-6. [DOI] [PubMed] [Google Scholar]
- 388.Liu Z, Zhang G, Li J, Liu J, Lv P. The tumor-suppressive microRNA-135b targets c-myc in osteoscarcoma. PLoS One. 2014;9:e102621. doi: 10.1371/journal.pone.0102621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 389.Benassi B, Flavin R, Marchionni L, Zanata S, Pan Y, Chowdhury D, Marani M, Strano S, Muti P, Blandino G, Loda M. MYC is activated by USP2a-mediated modulation of microRNAs in prostate cancer. Cancer discovery. 2012;2:236–247. doi: 10.1158/2159-8290.CD-11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Lin F, Ding R, Zheng S, Xing D, Hong W, Zhou Z, Shen J. Decrease expression of microRNA-744 promotes cell proliferation by targeting c-Myc in human hepatocellular carcinoma. Cancer cell international. 2014;14:58. doi: 10.1186/1475-2867-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Zhen Y, Liu Z, Yang H, Yu X, Wu Q, Hua S, Long X, Jiang Q, Song Y, Cheng C, Wang H, Zhao M, Fu Q, Lyu X, Chen Y, Fan Y, Liu Y, Li X, Fang W. Tumor suppressor PDCD4 modulates miR-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell death & disease. 2013;4:e872. doi: 10.1038/cddis.2013.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Yamada Y, Hidaka H, Seki N, Yoshino H, Yamasaki T, Itesako T, Nakagawa M, Enokida H. Tumor-suppressive microRNA-135a inhibits cancer cell proliferation by targeting the c-MYC oncogene in renal cell carcinoma. Cancer science. 2013;104:304–312. doi: 10.1111/cas.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Wang F, Xia J, Wang N, Zong H. miR-145 inhibits proliferation and invasion of esophageal squamous cell carcinoma in part by targeting c-Myc. Onkologie. 2013;36:754–758. doi: 10.1159/000356978. [DOI] [PubMed] [Google Scholar]
- 394.Shao Y, Qu Y, Dang S, Yao B, Ji M. MiR-145 inhibits oral squamous cell carcinoma (OSCC) cell growth by targeting c-Myc and Cdk6. Cancer cell international. 2013;13:51. doi: 10.1186/1475-2867-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Min DJ, Ezponda T, Kim MK, Will CM, Martinez-Garcia E, Popovic R, Basrur V, Elenitoba-Johnson KS, Licht JD. MMSET stimulates myeloma cell growth through microRNAmediated modulation of c-MYC. Leukemia. 2013;27:686–694. doi: 10.1038/leu.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Miao LJ, Huang SF, Sun ZT, Gao ZY, Zhang RX, Liu Y, Wang J. MiR-449c targets c-Myc and inhibits NSCLC cell progression. FEBS letters. 2013;587:1359–1365. doi: 10.1016/j.febslet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 397.Abe W, Nasu K, Nakada C, Kawano Y, Moriyama M, Narahara H. miR-196b targets c-myc and Bcl-2 expression, inhibits proliferation and induces apoptosis in endometriotic stromal cells. Hum Reprod. 2013;28:750–761. doi: 10.1093/humrep/des446. [DOI] [PubMed] [Google Scholar]
- 398.Yamamura S, Saini S, Majid S, Hirata H, Ueno K, Deng G, Dahiya R. MicroRNA-34a modulates c-Myc transcriptional complexes to suppress malignancy in human prostate cancer cells. PLoS One. 2012;7:e29722. doi: 10.1371/journal.pone.0029722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Takwi AA, Li Y, Becker Buscaglia LE, Zhang J, Choudhury S, Park AK, Liu M, Young KH, Park WY, Martin RC. A statin-regulated microRNA represses human c-Myc expression and function. EMBO molecular medicine. 2012;4:896–909. doi: 10.1002/emmm.201101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Nelson WG, De Marzo AM, Yegnasubramanian S. USP2a activation of MYC in prostate cancer. Cancer discovery. 2012;2:206–207. doi: 10.1158/2159-8290.CD-12-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Nadiminty N, Tummala R, Lou W, Zhu Y, Zhang J, Chen X, eVere White RW, Kung HJ, Evans CP, Gao AC. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J Biol Chem. 2012;287:1527–1537. doi: 10.1074/jbc.M111.278705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Liao JM, Lu H. Autoregulatory suppression of c-Myc by miR-185-3p. J Biol Chem. 2011;286:33901–33909. doi: 10.1074/jbc.M111.262030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Kappelmann M, Kuphal S, Meister G, Vardimon L, Bosserhoff AK. MicroRNA miR-125b controls melanoma progression by direct regulation of c-Jun protein expression. Oncogene. 2013;32:2984–2991. doi: 10.1038/onc.2012.307. [DOI] [PubMed] [Google Scholar]
- 404.Song J, Liu P, Yang Z, Li L, Su H, Lu N, Peng Z. MiR-155 negatively regulates c-Jun expression at the post-transcriptional level in human dermal fibroblasts in vitro: implications in UVA irradiation-induced photoaging. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2012;29:331–340. doi: 10.1159/000338488. [DOI] [PubMed] [Google Scholar]
- 405.Eades G, Yang M, Yao Y, Zhang Y, Zhou Q. miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. J Biol Chem. 2011;286:40725–40733. doi: 10.1074/jbc.M111.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Yang JJ, Tao H, Hu W, Liu LP, Shi KH, Deng ZY, Li J. MicroRNA-200a controls Nrf2 activation by target Keap1 in hepatic stellate cell proliferation and fibrosis. Cell Signal. 2014;26:2381–2389. doi: 10.1016/j.cellsig.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 407.van Jaarsveld MT, Helleman J, Boersma AW, van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH, Berns EM, Verweij J, Pothof J, Wiemer EA. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene. 2013;32:4284–4293. doi: 10.1038/onc.2012.433. [DOI] [PubMed] [Google Scholar]
- 408.Wei J, Zhang Y, Luo Y, Wang Z, Bi S, Song D, Dai Y, Wang T, Qiu L, Wen L, Yuan L, Yang JY. Aldose reductase regulates miR-200a-3p/141-3p to coordinate Keap1-Nrf2, Tgfbeta1/2, and Zeb1/2 signaling in renal mesangial cells and the renal cortex of diabetic mice. Free radical biology & medicine. 2014;67:91–102. doi: 10.1016/j.freeradbiomed.2013.10.811. [DOI] [PubMed] [Google Scholar]
- 409.Cheng X, Ku CH, Siow RC. Regulation of the Nrf2 antioxidant pathway by microRNAs: New players in micromanaging redox homeostasis. Free Radic Biol Med. 2013;64:4–11. doi: 10.1016/j.freeradbiomed.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 410.Inukai S, Slack F. MicroRNAs and the genetic network in aging. J Mol Biol. 2013;425:3601–3608. doi: 10.1016/j.jmb.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Li N, Muthusamy S, Liang R, Sarojini H, Wang E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mech Ageing Dev. 2011;132:75–85. doi: 10.1016/j.mad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 412.Smit-McBride Z, Forward KI, Nguyen AT, Bordbari MH, Oltjen SL, Hjelmeland LM. Age-dependent increase in miRNA-34a expression in the posterior pole of the mouse eye. Mol Vis. 2014;20:1569–1578. [PMC free article] [PubMed] [Google Scholar]
- 413.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wang X, Bonventre JV, Parrish AR. The aging kidney: increased susceptibility to nephrotoxicity. Int J Mol Sci. 2014;15:15358–15376. doi: 10.3390/ijms150915358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Lavrovsky Y, Chatterjee B, Clark RA, Roy AK. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp Gerontol. 2000;35:521–532. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]