Figure 1.
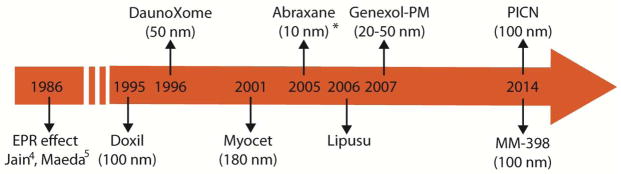
Chronology of first clinical approvals of nanomedicines after the introduction of the EPR effect. Myocet is approved only in Europe and Canada, Lipusu in China, Genexol-PM in South Korea and PICN in India. The size of nanoparticles given in parenthesis is approximate. *Abraxane becomes ~10 nm in size from ~130 nm following disintegration in the blood 17. The size of Lipusu is not available.
