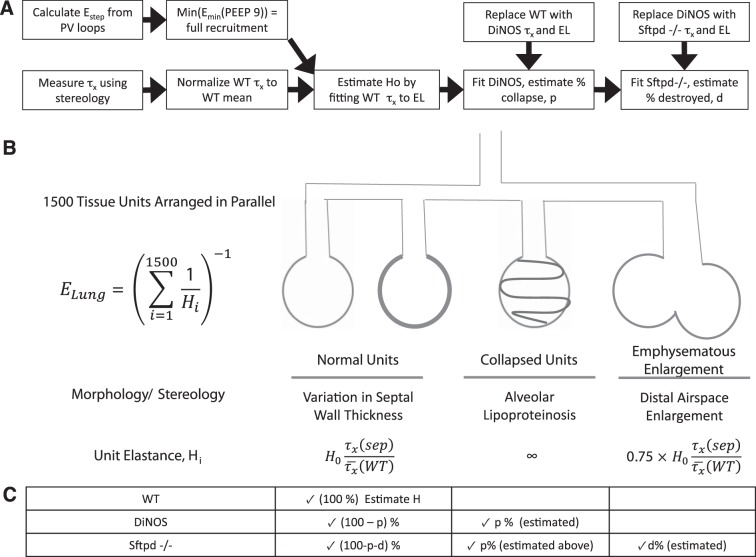
Fig. 1.
Schematic diagram of model structure and workflow. A: integration of stereological and mechanical measurements is used to estimate contribution of acinar mechanics with progressive increase in model complexity. Pressure-volume loops from wild-type (WT) mice are used to determine the elastance of the fully recruited lung. Using normalized stereological measurements of septal thickness [τx/τx—(WT)], the tissue elastance scaling constant H0 is estimated by least-squares minimization. The value of H0 represents the intrinsic stiffness of an ideal unit, from which the entire tissue elastance distribution can be simulated for each genotype only by changing [τx/τx—(WT)]. In the double-knockout (DiNOS) mouse, lipoproteinosis was modeled by allowing a fraction of ventilatory units, p, to have infinite elastance, mimicking stochastic alveolar collapse. This percentage was estimated by single variable optimization on p to minimize error between simulation and measured EL spectra. The surfactant protein D (SP-D)-deficient (Sftpd−/−) mouse was modeled as having acinar collapse equivalent to the DiNOS mouse but with stochastic loss of acinar structure due to emphysematous change. A fraction of acini, d, was estimated with single variable optimization to have 75% of the predicted elastance, reflecting the loss of total wall surface area measured stereologically in Table 2. B: schematic representation of parallel structures in the modeled lung with their morphological and sterological correlates. C: fractions of acini with each mechanical behavior are shown for WT, DiNOS, and Sftpd−/− mice, demonstrating increasing model complexity with additional stereological features. The quantity estimated using the elastance spectra of each genotype is explicitly shown.