Abstract
Gastric ulcers affect many people around the world and their development is a result of the imbalance between aggressive and protective factors in the gastric mucosa. Scutia buxifolia, commonly known as coronilha, has attracted the interest of the scientific community due to its pharmacological properties and its potential therapeutic applications. In this study, the preventive effects of the crude extract of Scutia buxifolia (ceSb) against gastric ulcer induced by 70% ethanol were evaluated in male Wistar rats. In addition, the composition of ceSb was clarified by high-performance liquid chromatography (HPLC). S. buxifolia extract (100, 200 and 400 mg/kg body weight) attenuated oxidative and histopathological features induced by ethanol. Moreover, all evaluated doses of ceSb caused significant (P<0.001 and P<0.0001) and dose-dependent increase in sulfhydryl groups (NPSH) levels, catalase (CAT) and superoxide dismutase (SOD) activities. Furthermore, the administration of ceSb reversed the increase in lipid peroxidation produced by ethanol. The protective effect of the extract could be attributed to antioxidant compounds present in the ceSb, such as flavonoids and phenolic acids, which were quantified by HPLC. Thus, an antioxidant effect of the extract leads to a protection on gastric tissue. These results indicate that S. buxifolia could have a beneficial role against ethanol toxicity by preventing oxidative stress and gastric tissue injury.
KEY WORDS: Scutia buxifolia, Antioxidant, Gastric ulcer, HPLC
Graphical abstract
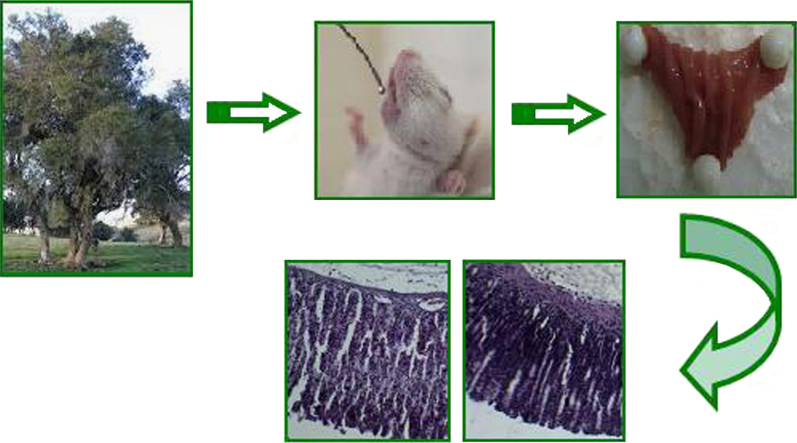
The crude extract of Scutia buxifolia (100, 200 and 400 mg/kg) protected the gastric mucosa of rats against oxidative damage caused by ethanol administration; these effects were attributed due to the antioxidant properties of the species.
1. Introduction
Gastric ulcer is one of the major gastrointestinal disorders, which occurs due to an imbalance between the offensive (gastric acid secretion) and defensive (gastric mucosal integrity) factors1, 2. The incidence of peptic ulcer is increased due to stress, smoking, alcohol, Helicobacter pylori and ingestion of non-steroidal anti-inflammatory drugs (NSAID) 3, 4, 5. It has been suggested that reactive oxygen species (ROS), primarily super-oxide anions, hydroxyl radicals, and lipid peroxides, are the harmful species known to cause the gastric ulcer development6. To scavenge ROS, gastric cell have several enzymatic and non-enzymatic antioxidants including catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), endogenous glutathione (GSH) and sulfhydryl groups (NPSH), but excessive generation of ROS enhance lipid peroxidation and depletes these antioxidants enzymes7, 8, 9.
There are many different experimental models of gastric ulcer induction, including ethanol and acetic acid2. Using such animal models, researchers simulate conditions to which humans may be exposed and, as a result, develop gastric ulcers. Ethanol is known as a cause of gastric damage by altering protective factors, including decreasing mucus production and blood circulation within the mucosa4, 10. In addition, the gastric damage caused by ethanol may be due to the generation of reactive species, decreased cell proliferation, and an exacerbated inflammatory response10, 11, 12.
The prevention or cure of peptic ulcers is one of the most important challenges confronting medicine nowadays, as it is certainly a major human illness affecting nearly 8%–10% of the global population, of which 5% suffer from gastric ulcers13. Gastric ulcer therapy faces a major drawback because most of the drugs currently available in the market show limited efficacy against gastric diseases and are often associated with severe side effects14, 15.
Controlling the formation of reactive species and secretion of gastric acid are essential for the treatment of these pathologies. In this context, medicinal plants containing a wide variety of antioxidants, such us phenolic acids, flavonoid, coumarins, tannins and terpenoids compounds, are some of the most attractive sources of new drugs and have been shown to produce promising results in the treatment of gastric ulcers16, 17, 18, 19.
Scutia buxifolia Reissek (Rhamnaceae), popularly known in Brazil as “coronilha”, is native tree from South America, with a dispersion area that comprises Rio Grande do Sul State in Brazil, and the countries Argentina and Uruguay. In these regions, an aqueous infusion prepared with stem bark of S. buxifolia has been described and widely used in folk medicine for cardiotonic, diuretic and antihypertensive properties20, 21. Phytochemical screening of S. buxifolia fractions revealed the presence of cyclopeptide alkaloids, steroids, polyphenols and flavonoids22, 23, 24, 25. Among the studies that were conducted, alkaloids isolated from S. buxifolia displayed in vitro antimicrobial activity22, 26. Cytotoxicity effects of extracts from leaves, twigs and stem bark of the plant were evaluated by the Artemia salina assay, as well as the antimicrobial, antimycobacterial and antiviral activities23, 27, 28. Furthermore, de Freitas et al.29 showed that the lyophilized aqueous extract of the stem bark of S. buxifolia did not cause hepatotoxicity. Extracts from the leaves and stem bark of S. buxifolia were effective inhibitors of TBARS production and also presented DPPH scavenger activity, while polyphenols and flavonoids were associated with this properties, indicating that this plant have promising compounds to be tested as potential drugs for the treatment of diseases resulting from oxidative stress25.
The aim of the present study was to evaluate the protective effect of S. buxifolia crude extract against toxicity of ethanol on gastric mucosal by evaluating oxidative stress markers, antioxidant defense along with morphological and histopathological damage. In order to clarify the properties of the crude extract of S. buxifolia (ceSb), the extract composition were also evaluated by high-performance liquid chromatography (HPLC).
2. Experimental
2.1. Chemicals, apparatus and general procedures
Methanol, ethanol, acetic acid, gallic acid, chlorogenic acid and caffeic acid were purchased from Merck (Darmstadt, Germany). Quercetin, rutin, kaempferol and omeprazole were acquired from Sigma Chemical Co. (St. Louis, MO, USA). Milli-Q ultra-purified water was used in preparing the samples. High performance liquid chromatography-diode array detection (HPLC-DAD) was performed with a Shimadzu Prominence Auto Sampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector and LC solution 1.22 SP1 software.
2.2. Plant collection and extract preparation
Stem bark of S. buxifolia were collected on October of 2007 in the first district of the council of Dom Pedrito, in the Rio Grande do Sul State, Brazil (coordinates 30°59′09″ S and 54°27′44″ W). Voucher specimen was archived in the herbarium of Department of Biology at Federal University of Santa Maria, register number SMBD 10919. The stem bark were dried at room temperature and powdered in a knife mill (0.86 µm), resulting in a mass of 651.52 g of plant material, which was submitted to maceration at room temperature with ethanol 70% for a week with daily shake. After filtration, the extract was evaporated under reduced pressure to remove the ethanol. Then, the extract was stored and subjected to a slow evaporation of the water fraction of the solvent in an oven, for future use of the remaining solids (ceSb).
2.3. Determination of total phenolics contents
The determination of total phenolic contents in ceSb was determined by the Folin–Ciocalteu method with slightly modifications30. The samples were read at 730 nm in spectrophotometer. Gallic acid in the range of 0.005–0.030 mg/mL was used as a standard phenol, giving the calibration equation: Y=11.969X−0.0454 (r=0.9987). Test was carried out in triplicate and the result was expressed in milligrams equivalents of gallic acid (GAE) per gram of crude extract.
2.4. Determination of total flavonoids content
The flavonoid content in ceSb was determined based on the formation of flavonoid-aluminum complex31. One milliliter of sample was mixed with 1 mL of 2% aluminum chloride solution. After incubation for 15 min at room temperature, the absorbance of the reaction mixture was measured at 420 nm. A standard curve was first plotted using quercetin (0.012–0.200 mg/mL) as a standard, giving the calibration equation: Y=0.0045X−0.014 (r=0.9992). The amount of flavonoids was expressed as quercetin mg/g dry crude extract and all tests were carried out in triplicate.
2.5. Determination of total tannins content
The tannins content in ceSb was performed using the method described by Morrison et al.32 Samples in concentrations of 0.25 mg/mL, 5 mL of solution A (1 g vanillin in 100 mL of methanol) and solution B (8 mL HCl in 100 mL of methanol) were used to experiment. The samples were read at 500 nm in spectrophotometer. The total tannins content was expressed in milligrams equivalents of catechin per gram of each fraction. The equation obtained for the calibration curve of catechin in the range of 0.001–0.025 mg/mL was Y=0.00015X+0.005 (r=0.9979). The experiments were conducted in triplicate.
2.6. Determination of total alkaloids content
The alkaloids content in ceSb (20 mg/mL) was determined using the method described by Sreevidja and Mehrotra33, where Dragendorff׳s reagent precipitates alkaloids in plants materials. It is based on the formation of yellow bismuth complex in nitric acid medium with thiourea. Mixture of thiourea and nitric acid were used as a blank. The samples were read at 435 nm in spectrophotometer. The equation obtained for the calibration curve of bismuth nitrate pentahydrate solution in the range of 0.01–0.09 mg/mL was Y=2.2783X+0.0361 (r=0.9997). The experiments were conducted in triplicate.
2.7. HPLC/DAD analyses of S. buxifolia extract composition
Reverse phase chromatographic analyses were carried out under gradient conditions using C18 column (250 mm×4.6 mm) packed with 5 μm diameter particles; the mobile phase was water containing 2% acetic acid (A) and methanol (B), and the composition gradient was: 5% (B) for 2 min; 25% (B) until 10 min; 40%, 50%, 60%, 70% and 80% (B) every 10 min; following the method described by Amaral et al.12 with slight modifications. The ceSb and mobile phase were filtered through 0.45 μm membrane filter (Millipore) and then degassed by ultrasonic bath prior to use, the extract was analyzed dissolved in methanol at a concentration of 8 mg/mL. Stock solutions of standards references were prepared in methanol at a concentration range of 0.031–0.250 mg/mL for kaempferol, quercetin and rutin, and 0.006–0.250 mg/mL for gallic, chlorogenic and caffeic acids. Quantification was carried out by integration of the peaks using the external standard method, at 254 nm for gallic acid, 325 nm for caffeic and chlorogenic acids, and 365 nm for quercetin, rutin and kaempferol. The flow rate was 0.8 mL/min and the injection volume was 40 μL. The chromatography peaks were confirmed by comparing their retention time and Diode-Array-UV spectra with those of the reference standards. All chromatography operations were carried out at ambient temperature and in triplicate. The respective standard solutions calibrations curves were Y=53985X+1020.6 (r=0.9859) for gallic acid; Y=52548X+1082.3 (r=0.9850) for chlorogenic acid; Y=87846X+1093.0 (r=0.9938) for caffeic acid; Y=103861X−1235.8 (r=0.9921) for rutin; Y=150833X−4741.7 (r=0.9949) for quercetin and Y=130745X−1897.9 (r=0.9928) for kaempferol.
The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the responses and the slope using three independent analytical curves, as defined by Sabir et al.34, LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve.
2.8. Animals
Male Wistar rats (200–250 g), obtained from the General Animal House of the Federal University of Santa Maria, were kept on a separate animal room, in a 12-h light/dark cycle at room temperature and were fasted 16 h with free access to water before the experiment. All animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources from Federal University of Santa Maria, Brazil (013/2012).
2.8.1. The experimental protocol and ethanol-induced gastric lesions method
The animals were randomly divided into 10 groups (A–J), with six animals each. Five groups of animals received distillated water as vehicle (0.5 mL/100 g body weight) and the other five groups received a 70% aqueous solution (v/v) of ethanol by oral gavage (0.5 mL/100 g body weight). After 1 h of ethanol or vehicle administration, the animals received ceSb intragastrically at doses of 0 g/kg (vehicle), 100 mg/kg, 200 mg/kg and 400 mg/kg body weight, the group positive control received omeprazole 30 mg/kg body weight. The chosen model of gastric damage induced by ethanol has already been described35. The S. buxifolia extract doses used were adapted from a previous study of toxicity29.
The treatment groups and experimental protocol are detailed below:
Group A – control group: received only distilled water (0.5 mL/100 g body weight).
Group B – ethanol group: received only 70% ethanol (0.5 mL/100 g body weight).
Group C – omeprazole control group: received distilled water (0.5 mL/100 g body weight), 1 h after omeprazole (30 mg/kg body weight).
Group D – omeprazole+ethanol group: received 70% ethanol (0.5 mL/100 g body weight), 1 h after omeprazole (30 mg/kg body weight).
Group E – 100 mg/kg ceSb control group: received distilled water (0.5 mL/100 g body weight), 1 h after ceSb (100 mg/kg body weight).
Group F – 100 mg/kg ceSb+ethanol group: received 70% ethanol (0.5 mL/100 g body weight), 1 h after ceSb (100 mg/kg body weight).
Group G – 200 mg/kg ceSb control group: received distilled water (0.5 mL/kg body weight), 1 h after ceSb (200 mg/kg body weight).
Group H – 200 mg/kg ceSb+ethanol group: received 70% ethanol (0.5 mL/100 g body weight), 1 h after ceSb (200 mg/kg body weight).
Group I – 400 mg/kg ceSb control group: received distilled water (0.5 mL/100 g body weight), 1 h after ceSb (400 mg/kg body weight).
Group J – 400 mg/kg ceSb+ethanol group: received 70% ethanol (0.5 mL/100 g body weight), 1 h after ceSb (400 mg/kg body weight).
One hour after ceSb or omeprazole administration, the animals were euthanized by deep anesthesia induced by thiopental at 100 mg/kg body weight, administered intraperitoneally. The stomachs were immediately removed, washed with saline solution (0.9% NaCl) and the glandular portion was separated for macroscopic evaluation (gastric lesion index). Afterwards, a portion of gastric tissue was collected for histopathological analysis and the remained tissue was homogenized in 9 volumes of 0.1 mol/L potassium phosphate buffer (pH 7.4) using a Polytron mixer (Kinematica AG, Switzerland). The homogenate was centrifuged at 3000×g at 4 °C for 10 min to yield a low-speed supernatant that was used to measure the biochemical parameters.
2.8.2. Macroscopic evaluation
The stomachs were opened along the greater curvature and washed with 0.9% NaCl and examined by a blinded pathologist for macroscopic lesions in the glandular part under a dissecting microscope. The severity of macroscopic lesions formed were estimated using an ulcer index as previously reported36, 37 using the following scale: 0=normal mucosa; 1=hyperemic mucosa up to 3 small patches; 2=4–10 small patches; 3=more than 10 small or up to 3 medium-sized patches; 4=4 to 6 medium-sized patches; 5=more than 6 medium-sized or up to 3 large patches; 6=4–6 large patches; 7=7–10 large patches; 8=more than 10 large patches; and 10=large patches of extensive necrotic zones. A “small patch” is defined as an area of lesion up to 2 mm across (maximum diameter), a “medium-sized patch” as between 2 and 4 mm across, and a “large patch” as more than 4 mm across. For hemorrhage, petechiae (1 or more small red dot), edema and loss of mucus (Alcian Blue solution, 0.1%, w/v, in 0.16 mmol/L sucrose solution, was used as dye), the stomachs with no injuries received score 0, stomachs with minor injury received score +1, those with moderate and severe injuries were given a score of +2 and +3, respectively38.
2.8.3. Histopathological examinations
For microscopic analysis, a portion of stomach from each experimental group was fixed in 10% formalin and immersed in paraffin. Sections of 5 mm were obtained with a standard microtome and were stained with hematoxylin and eosin37. The sections were examined by a pathologist without knowledge of the experimental groups for presence of any negative features, such as edema, erosion, ulceration and necrosis. The severity of histopathological changes was quantified according to an arbitrary scale as described before, with some modifications39. Gastric tissue with no negative features was given a score of 0. Gastric tissue with mild histopathological damage was given a score of +1. Those with moderate and severe negative features were given a score of +2 and +3, respectively. Results were expressed as a histopathological score.
2.8.4. Thiobarbituric acid reactive substances (TBARS)
Stomach tissue lipoperoxidation (LPO) estimation was performed using the TBARS assay as previously described, where the colorimetric reaction of the LPO product malondialdehyde (MDA) with thiobarbituric acid (TBA) is quantified. The concentration of TBA reactive substances was measured at 532 nm using a standard curve of MDA, and the results were expressed as nmol MDA/mg protein40.
2.8.5. Non-enzimatic antioxidant defense
Tissue non-protein sulfhydryl groups (NPSH) were quantified after mixing the homogenate with 10% trichloroacetic acid (1:1, v/v), followed by centrifugation, as described by Ellman41. Cysteine was used for preparation of a standard curve.
2.8.6. Catalase activity (CAT) assay
CAT activity was determined by measuring the decrease in hydrogen peroxide (H2O2) absorption at 32 °C. The method is based on the consumption of H2O2 by CAT and loss of absorbance at 240 nm42.
2.8.7. Superoxide dismutase activity (SOD) assay
Superoxide dismutase is an enzyme which catalyzes the dismutation of superoxide radical to form hydrogen peroxide and oxygen. The assay for determination of indirect SOD-activity is based in the inhibition of reaction between superoxide radical with adrenaline43.
2.8.8. Protein quantification
The amounts of LPO were normalized to the amount of stomach protein content. The quantification of the protein was performed following Lowry method44, where the maximum absorbance for the solution of Folin–Ciocalteu due to its interaction to bovine serum albumin (BSA) protein, occurs at 625 nm.
2.9. Statistical analysis
The results were expressed as mean±standard deviation (SD). Statistical comparisons were performed by one-way analysis of variance followed Tukey׳s post-hoc test. The data were analyzed by using Statistical Package for the Social Sciences (SPSS, version 18.0). A P-value less than 0.01 were considered to be significant different.
3. Results
3.1. Total phenols, flavonoids, tannins and alkaloids contents
The quantitative phytochemical results showed the presence of phenolics (141.09±0.71 mg GAE/g of extract), flavonoids (100.37±0.56 mg quercetin/g of extract), tannins (66.67±0.17 mg catechin/g extract) and alkaloids (1.59±0.08 mg alkaloids/g extract) (Table 1).
Table 1.
Content of phenolics, flavonoids, tannins and alkaloids in crude extract of S. buxifolia.
| S. buxifolia compounds | Quantities |
LOD (μg/mL) | LOQ (μg/mL) | |
|---|---|---|---|---|
| (mg/g) | (%) | |||
| Total phenolics ⁎ | 141.09±0.71 | – | – | – |
| Total flavonoids # | 100.37±0.56 | – | – | – |
| Total tannins ‡ | 66.67±0.17 | – | – | – |
| Total alkaloids | 1.59±0.08 | – | – | – |
| Gallic acid | 41.3a±0.22 | 4.13 | 0.017 | 0.056 |
| Chlorogenic acid | 19.2a±0.19 | 1.92 | 0.008 | 0.025 |
| Caffeic acid | 77.5a±0.03 | 7.75 | 0.023 | 0.075 |
| Rutin | 8.9a±0.34 | 0.89 | 0.010 | 0.032 |
| Quercetin | 90.3a±0.05 | 9.03 | 0.009 | 0.029 |
| Kaempferol | 5.4a±0.15 | 0.54 | 0.032 | 0.104 |
LOD: limit of detection; LOQ: limit of quantification.
Results are expressed as mean±standard deviations (SD) of three determinations.
Gallic acid equivalent.
Quercetin equivalent.
Catechin equivalent.
P<0.01, mean values differ by the Tukey test.
3.2. HPLC/DAD analysis
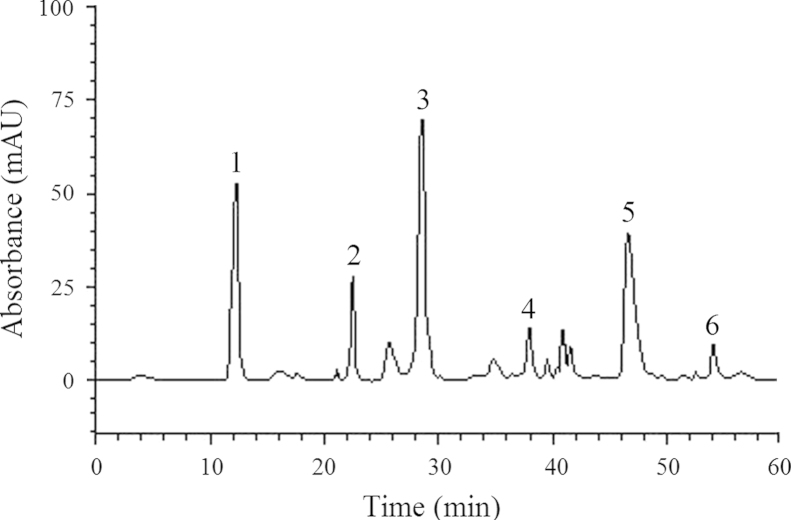
HPLC fingerprinting of ceSb revealed the presence of the gallic acid (retention time-tR 12.4 min; peak 1; 4.13%), chlorogenic acid (tR=23.1 min; peak 2; 1.92%), caffeic acid (tR=28.6 min; peak 3; 7.75%), rutin (tR=37.5 min; peak 4; 0.89%), quercetin (tR=47.6 min; peak 5; 9.03%) and kaempferol (tR=54.9 min; peak 6; 0.54%) (Fig. 1 and Table 1). The HPLC analysis revealed that flavonoids (quercetin, rutin and kaempferol) and phenolics acids (gallic, chlorogenic, caffeic acids) are the major components of the extract.
Figure 1.
High performance liquid chromatography (HPLC) chromatogram of Scutia buxifolia crude extract. Gallic acid (peak 1), chlorogenic acid (peak 2), caffeic acid (peak 3), rutin (peak 4), quercetin (peak 5) and kaempferol (peak 6).
3.3. Macroscopic analysis
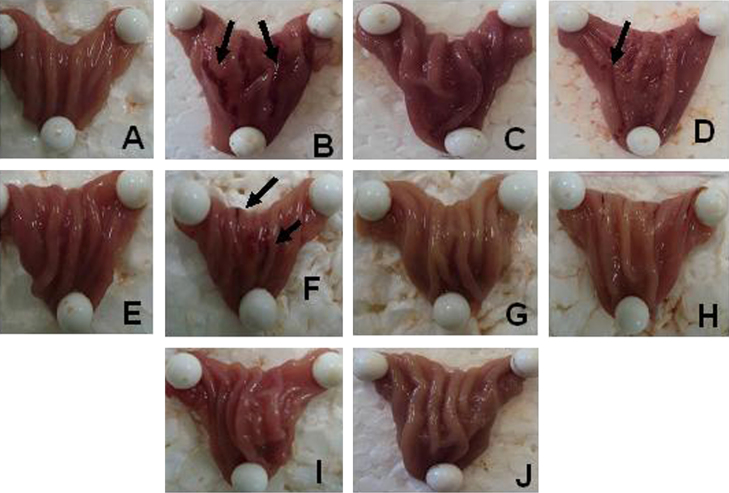
The assay revealed a significant effect of ethanol on gastric tissue (P<0.01; Figure 2, Figure 3). The animals that received 70% ethanol developed a consistent macroscopic damage which were evidenced by presence of ulceration hemorrhagic (Fig. 2B). It is attenuated by the administration of omeprazole (30 mg/kg) with a few fields of hyperemia (Fig. 2D). In addition, the ceSb did not show any macroscopic toxicity, preserving the morphological integrity of the gastric mucosa (Fig. 2E, G and I) when compared to non-treated control group (Fig. 2A). Furthermore, the animals treated with ceSb at 200 and 400 mg/kg were able to reversed the damage induced by ethanol (Fig. 2H and J, respectively), with very similar aspect to the control group (Fig. 2A).
Figure 2.
Demonstrative images of stomachs from all experimental groups. Observe images from control group (A), ethanol group (B) omeprazole control group (C), omeprazole+ethanol group (D), 100 mg/kg ceSb control group (E), 100 mg/kg ceSb+ethanol group (F), 200 mg/kg ceSb control group (G), 200 mg/kg ceSb+ethanol group (H), 400 mg/kg ceSb control group (I) and 400 mg/kg ceSb+ethanol group (J).
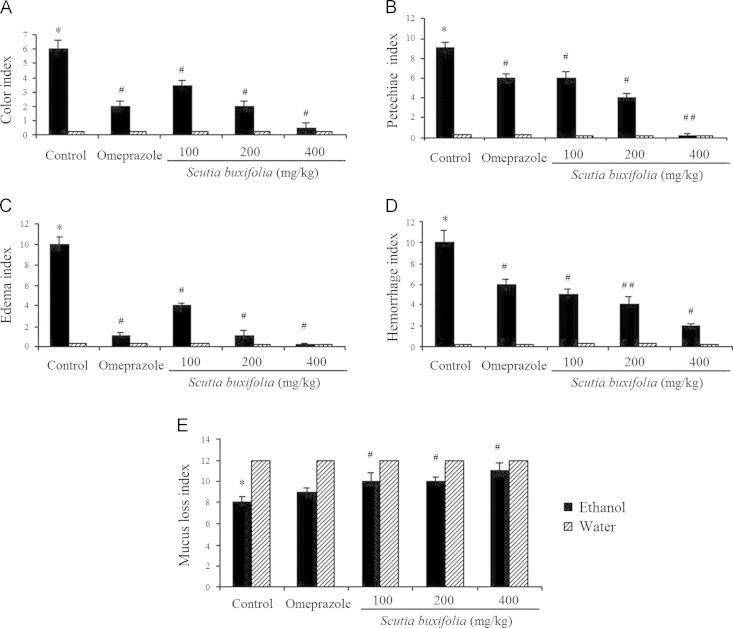
Figure 3.
Color (A), petechiae (B), edema (C), hemorrhage (D) and mucus loss (E) indexes (magnification of 10×) of stomach from rats treated with ethanol and/or omeprazole or Scutia buxifolia extract. Data are means+SD (n=6). Significant difference when compared to water control group (*P<0.01); significant difference when compared to ethanol control group (#P<0.01, ##P<0.001).
Post-hoc comparisons demonstrated that 1 h exposure to ethanol is able to cause injury to gastric tissue characterized by macroscopic features such as discoloration (P<0.01; Fig. 3A), petechiaes (P<0.01; Fig. 3B), edema (P<0.01; Fig. 3C), hemorrhage (P<0.01; Fig. 3D) and mucus loss (P<0.01; Fig. 3E). Although ceSb at dose of 200 and 400 mg/kg totally reversed all macroscopic lesions induced by ethanol (P<0.01; Figure 2, Figure 3), the dose of 100 mg/kg completely restored just edema and mucus loss occurrence (P<0.01; Fig. 3C and E). Moreover, ceSb at dose of 100 mg/kg partially ameliorated color, petec and hemorrhage (P<0.01; Fig. 3A, B and D). The omeprazole completely reversed the color and edema induced by ethanol (P<0.01; Fig. 3A and C).
3.4. Histopathology
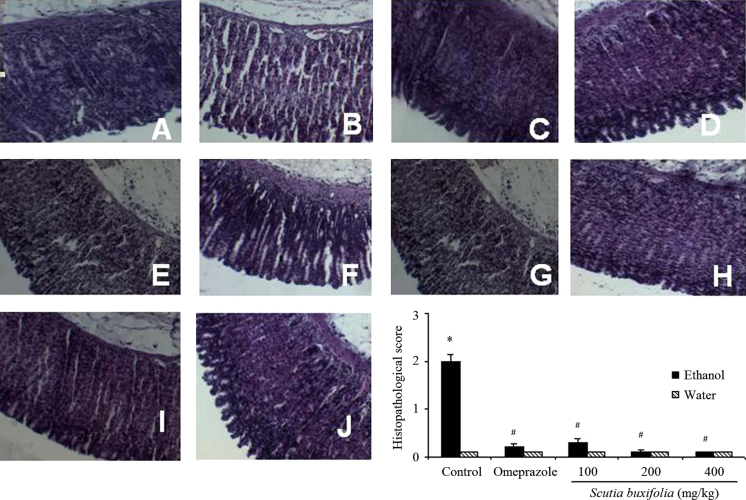
Acute exposure of rats to ethanol caused mucosal necrosis, edema and congestion along with inflammatory process characterized by neutrophils infiltration, as demonstrated by the histopathological score (P<0.01; Fig. 4B and L). These results confirm that ethanol causes gastric damage also at a microscopic level. Post-treatment with S. buxifolia extract (100, 200 and 400 mg/kg) ameliorated injuries caused by ethanol (P<0.001; Fig. 4F, H and J, respectively, and Fig. 4L) and did not induce any damage to gastric tissue per se (Fig. 4E, G and I).
Figure 4.
Representative histology (magnification of 100×; A–J) and histopathological damage score of gastric tissue from animals treated with ethanol and/or Scutia buxifolia extracts (L). Control group (A), ethanol group (B), omeprazole control group (C), omeprazole+ethanol group (D), 100 mg/kg ceSb control group (E), 100 mg/kg ceSb+ethanol group (F), 200 mg/kg ceSb control group (G), 200 mg/kg ceSb+ethanol group (H), 400 mg/kg ceSb control group (I) and 400 mg/kg ceSb+ethanol group (J). In panel L, the score 0 indicates absence of negative features (edema, erosion, ulceration and necrosis) while score 3 indicates severe negative features. Data are means±SD (n=5–6). Significant difference when compared to water control group (*P<0.01); significant difference when compared to ethanol control group (#P<0.001).
3.5. Effect on lipid peroxidation
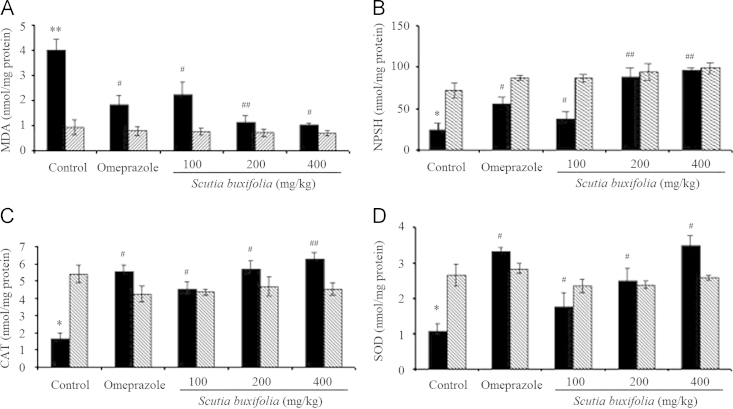
The ethanol group showed significant change on oxidative markers with an increase on lipid peroxidation when compared to control group (MDA=4.02±0.42 and 0.94±0.3 nmol/mg protein, P<0.001). However, the animals which received omeprazole at 30 mg/kg and ceSb at 100, 200 and 400 mg/kg completely attenuated the damage induced by ethanol (MDA=1.83±0.36, 2.25±0.47, 1.14±0.26 and 1.01±0.09 nmol/mg protein, respectively). Furthermore, the ceSb, at all doses tested, was able to significantly prevent the increase on lipid peroxidation in relation to respective control animals (P<0.001 and P<0.01) (Fig. 5A).
Figure 5.
Thiobarbituric acid reactive substances – TBARS (A), sulfhydryl groups – NPSH (B), CAT activity (C) and SOD activity (D) levels of gastric tissue from rats treated with ethanol and/or omeprazole or Scutia buxifolia extract. Data are means±SEM (n=5–9). Significant difference when compared to water control group (*P<0.01; **P<0.001). Significant difference when compared to ethanol control group (#P<0.01, ##P<0.001).
3.6. Effect on tissue NPSH
Ethanol caused a decreased NPSH content when compared to control group (25.56±8.4 and 72.45±9.1 nmol/mg protein, P<0.01; Fig. 5B). These results confirm the ability of ethanol in depleting antioxidant defenses. In addition, omeprazole (30 mg/kg) and ceSb extract (100, 200 and 400 mg/kg) restored NPSH levels (56.14±7.3, 39.05±9.11, 89.26±10.58 and 95.87±2.94 nmol/mg protein; P<0.001). However, the level of NPSH in stomach tissue was not affected by the treatment only with ceSb at different dosages (100, 200 and 400 mg/kg ceSb control group), maintaining similar levels to the respectively control group (P<0.001; Fig. 5B).
3.7. Enzymatic antioxidant defense
Statistical analysis revealed a significant decrease in CAT and SOD activities in gastric tissue after ethanol administration (1.65±0.35 nmol/mg protein and 1.07±0.20 nmol/mg protein, respectively; P<0.01) when compared to control group. In addition, the omeprazole at 30 mg/kg group and ceSb at all doses tested were able to significantly reversed, dose-dependent manner, the decrease on CAT and SOD activities induced by ethanol in relation to animals from respective control groups (Fig. 5C and D).
4. Discussion
Phytochemical screening of the crude extract of S. buxifolia stem bark (ceSb) showed the presence of acids phenolics, flavonoids, tannins and alkaloids (Table 1). The large amount of total phenolics and flavonoids contents detected in ceSb can be attributed to the antioxidant potential formerly described for this species25. In addition, HPLC analysis revealed that gallic acid, chlorogenic acid, caffeic acid, quercetin, rutin and kaempferol are the main compounds present in ceSb (Fig. 1 and Table 1), all found substances are well-known antioxidants. These compounds scavenge the free radical and play an important role in the prevention and therapy of diseases. Gallic acid, caffeic acid, rutin and quercetin are strong natural antioxidant, decrease the peroxidation and have anti-ulcerogenic, anti-mutagenic and anti-cancerogenic properties14, 17, 45, 46.
Acute exposure of the gastric mucosa of rats to ethanol can result in gastric lesions similar to those occurring in gastric ulcer; hence, ethanol-induced gastric ulcers have been widely used for the evaluation of gastroprotective activity4, 10. Accordingly, it was observed that ethanol administration to rats caused macroscopic lesions to gastric tissue, such as loss of normal color and mucus along with presence of petechiae, hemorrhage and edema (Figs. 2B and 3). These lesions are most likely related to mucus depletion and a constrictive effect on veins and arteries of the gastric mucosal, producing congestion, inflammation and tissue injury8. The reduction of gastric mucosal blood flow can result in hemorrhage and necrosis in damaged tissue5, 14.
In order to confirm the results of antiulcer experiment, the stomachs were also evaluated by histopathological examination (Fig. 4). In histological observation, the stomach of control animals showed no damage (Fig. 4A). However, rats 1 h after of exposure to ethanol presented damage to gastric tissue at a microscopic level. Histopathological injury caused by ethanol administration is characterized by edema and congestion of mucosal, as well as inflammatory process characterized by neutrofils infiltration (Fig. 4B). However, ceSb was able to reverse the damage caused by ethanol, probably exert potent antiinflammatory effect in gastric mucosa. This activity can be confirmed by microscopic evidence obtained in our analysis, decreasing the infiltration of inflammatory cells (neutrophils) (Fig. 4F, H and J) in relation to samples from stomachs of rats that received ethanol-only (Fig. 4B). Furthermore, the ceSb at 200 and 400 mg/kg was able to protect the histological structure of the gastric mucosa, preventing swelling (Fig. 4H and J, respectively) and preventing the infiltration of inflammatory cells (neutrophils) at 100, 200 and 400 mg/kg (Fig. 4F, H and J, respectively).
The abnormal elevation of reactive species corresponds to one of the main aggressive mechanisms of ethanol, which can cause gastric cell damage and death4, 12. In this study, ethanol induced depletion of non-enzymatic defenses (NPSH groups) and inhibition of the antioxidant enzyme CAT and SOD. In fact, depletion of glutathione (the major non-protein thiol) and inhibition of CAT and SOD after ethanol exposure has already been described5, 8, 46, and is directly involved in increased lipid peroxidation observed in ethanol-treated rats. Besides, lipid peroxidation in gastric tissue plays a significant role in the pathogenesis of ethanol-induced gastric lesions10, 11, 47. Previous reports confirm that ethanol increases superoxide anion and hydroxyl radical production by neutrophils and these ROS cause LPO in the gastric mucosa and tissue damage1.
S. buxifolia extract restored, in a dose dependent manner, the gastric mucosal damage and oxidative stress induced by ethanol (Fig. 5A–D). The broad antioxidant properties of ceSb were demonstrated by decreased levels of MDA and increase of antioxidant defenses (NPSH, SOD and CAT). These protective effects described for the crude extract of S. buxifolia can be associated with the presence of phenolic acids, mainly gallic, chlorogenic and caffeic acids, besides the flavonoids, such as quercetin, rutin and kaempferol in ceSb. The free radical scavenging activity of the ceSb25 might be considered as one of the possible mechanisms of its gastroprotective effect observed. Because oxygen derived radicals and agents with antioxidant properties have been implicated in the pathogenesis of ethanol-induced gastric ulcers48. In agreement with our findings, high levels of flavonoids also have already been found in the ethyl acetate fraction of S. buxifolia by Boligon et al.25. Similar results were also obtained in related to antioxidant enzyme activities by Alimi et al.47 and Liu et al.9.
Several studies have associated the protection of gastric ulcer to the presence of phenolic acid and flavonoids in plant extracts7, 8, 12, 16, 47. Hussain et al.17 described the significant gastroprotective effect of rutin by scavenging the ROS produced by gastric damage. Furthermore, quercetin and kaempferol also showed protective effects in ethanol-induced gastric ulcer by decreasing oxidative stress and increasing antioxidant enzyme activity15, 46. Flavonoids are antioxidant compounds that efficiently remove superoxide anion, hydroxyl, peroxyl and alcoxyl radicals8, while the removal of these same ROS along with peroxynitrite radicals has been described also for chlorogenic and caffeic acids12. Since superoxide anion and hydroxyl radical are the ROS involved in oxidative stress caused by ethanol and peroxyl and alcoxyl radicals are the major products of the LPO process1, 14, the scavenging of these species explains the protective effects of S. buxifolia against gastric injury induced by ethanol. In addition, some flavonoids also interfere in inflammation process and increase mucus content in gastric mucosal, resulting in cytoprotective effects2, 12.
In conclusion, ceSb (100, 200 and 400 mg/kg) were demonstrated gastric mucosal protection against oxidative injuries caused by ethanol and this protection is most likely due to antioxidant properties of S. buxifolia. In addition, the presence of phenolic acids and flavonoides in ceSb certainly contribute to the antiulcerogenic activity described here.
Acknowledgments
The authors wish to express gratitude to V. Batista for the collect of S. buxifolia in his property and to Prof. N.C.B. Záchia for the identification of the plant. The authors thank the financial support of Conselho Nacional de Desenvolvimento Cientifico e Tecnológico/Fundação de Amparo a Pesquisa do Rio Grande do Sul/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/Brazil.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Laine L., Takeuchi K., Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Shaker E., Mahmoud H., Mnaa S. Anti-inflammatory and anti-ulcer activity of the extract from Alhagi maurorum (camelthorn) Food Chem Toxicol. 2010;48:2785–2790. doi: 10.1016/j.fct.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Vonkeman H.E., Klok R.M., Postma M.J., Brouwers J.R., van de Laar M.A. Direct medical costs of serious gastrointestinal ulcers among users of NSAIDs. Drugs Aging. 2007;24:681–690. doi: 10.2165/00002512-200724080-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ineu R.P., Pereira M.E., Aschner M., Nogeueira C.W., Zeni G., Rocha J.B. Diphenyl diselenide reverses gastric lesions in rats: involvement of oxidative stress. Food Chem Toxicol. 2008;46:3023–3029. doi: 10.1016/j.fct.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Sowndhararajan K., Kang S.C. Protective effect of ethyl acetate fraction of Acacia ferruginea DC. against ethanol-induced gastric ulcer in rats. J Ethnopharmacol. 2013;148:175–181. doi: 10.1016/j.jep.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Smith G.S., Mercer D.W., Cross J.M., Barreto J.C., Miller T.A. Gastric injury induced by ethanol and ischemia-reperfusion in the rat. Dig Dis Sci. 1996;41:1157–1164. doi: 10.1007/BF02088232. [DOI] [PubMed] [Google Scholar]
- 7.Cadirci E., Suleyman H., Aksoy H., Halici Z., Ozgen U., Koc A. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem Biol Interact. 2007;170:40–48. doi: 10.1016/j.cbi.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 8.Mota C.S., Freitas R.B., Athayde M.L., Boligon A.A., Augusti P., Somacal S. Effect of Vernonia cognata on oxidative damage induced by ethanol in rats. Hum Exp Toxicol. 2011;30:675–684. doi: 10.1177/0960327110377646. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Tian X., Gou L., Fu X., Li S., Lan N. Protective effect of l-citrulline against ethanol-induced gastric ulcer in rats. Environ Toxicol Pharmacol. 2012;34:280–287. doi: 10.1016/j.etap.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Choi E., Hwang H., Kim I., Nam T. Protective effects of a polysaccharide from Hizikia fusiformis against ethanol toxicity in rats. Food Chem Toxicol. 2009;47:134–139. doi: 10.1016/j.fct.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Kaharaman A., Erkasap N., Köken T., Serteser M., Aktepe F., Erkasap S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicol. 2003;183:133–142. doi: 10.1016/s0300-483x(02)00514-0. [DOI] [PubMed] [Google Scholar]
- 12.Amaral G.P., de Carvalho N.R., Barcelos R.P., Dobrachinski F., Portella Rde L., da Silva M.H. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem Toxicol. 2013;55:48–55. doi: 10.1016/j.fct.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Calam J., Baron J.H. ABC of the upper gastrointestinal tract: pathophysiology of duodenal and gastric ulcer and gastric cancer. Br Med J. 2001;323:980–982. doi: 10.1136/bmj.323.7319.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandyopadhyay D., Biswas K., Bhattacharyya M., Reiter R.J., Banerjee R.K. Involvement of reactive oxygen species in gastric ulceration: protection by melatonin. Ind J Exp Biol. 2002;40:693–705. [PubMed] [Google Scholar]
- 15.Mota K.S.L., Dias G.E.N., Pinto M.E.F., Luiz-Ferreira A., Souza-Brito A.R., Hiruma-Lima C.A. Flavonoids with gastroprotective activity. Molecules. 2009;14:979–1012. doi: 10.3390/molecules14030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahida B., Abderrahman B., Nabil C. Antiulcerogenic activity of Zizyphus lotus (L.) extracts. J Ethnopharmacol. 2007;112:228–231. doi: 10.1016/j.jep.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Hussain M.T., Verma A.R., Vijayakumar M., Sharma A., Mathela C.S., Rao C.V. Rutin, a natural flavonoid, protects against gastric mucosal damage in experimental animals. Asian J Tradit Med. 2009;4:188–197. [Google Scholar]
- 18.Sathish R., Vyawahare R., Natarajan K. Antiulcerogenic activity of Lantana camara leaves on gastric and duodenal ulcers in experimental rats. J Ethnopharmacol. 2011;134:195–197. doi: 10.1016/j.jep.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 19.Gill N.S., Dhawan S., Jain A., Arora R., Bali M. Antioxidant and anti-ulcerogenic activity of wild Punica granatum ethanolic seed extracts. Res J Med Plant. 2012;6:47–55. [Google Scholar]
- 20.Wasicky R., Wasicky M., Joachimovits R. Erstuntersuchungen na Coronilha-Scutia buxifolia Reissek. Planta Méd. 1964;12:13–25. [Google Scholar]
- 21.Da Silva RCVAF, Crestani S., Souza P., Boligon A.A., Athayde M.L., Santos A.R. Endothelium-dependent and independent vasorelaxation induced by an n-butanolic fraction of bark of Scutia buxifolia Reiss (Rhamanaceae) J Ethnopharmacol. 2012;141:997–1004. doi: 10.1016/j.jep.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 22.Maldaner G., Marangon P., Ilha V., Caro M.S.B., Burrow R.A., Dalcol I.I. Cyclopeptide alkaloids from Scutia buxifolia Reiss. Phytochem. 2011;72:804–809. doi: 10.1016/j.phytochem.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Boligon A.A., Agertt V., Janovik V., Cruz R.C., Campos Marli M.A., Guillaume D. Antimycobacterial activity of the fractions and compounds from Scutia buxifolia. Rev Bras Farmacognosia. 2012;22:45–52. [Google Scholar]
- 24.Boligon A.A., Janovik V., Feltrin A.C., Machado M.M., Frohlich J.K., Athayde M.L. Phytoconstituents isolated from dichloromethane fraction of Scutia buxifolia Reissek stem bark. Latin Am J Pharm. 2010;29:450–453. [Google Scholar]
- 25.Boligon A.A., Pereira R.P., Feltrin A.C., Machado M.M., Janovik V., Rocha J.B. Antioxidant activities of flavonol derivatives from the leaves and stem bark of Scutia buxifolia Reiss. Bioresour Technol. 2009;100:6592–6598. doi: 10.1016/j.biortech.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 26.Morel A.F., Maldaner G., Ilha V., Missau F., Silva U.F., Dalcol I.I. Cyclopeptide alkaloids from Scutia buxifolia Reiss and their antimicrobial activity. Phytochem. 2005;66:2571–2576. doi: 10.1016/j.phytochem.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Boligon A.A., Janovik V., Frohlich J.K., Spader T.B., Froeder A.L., Alves S.H. Antimicrobial and cytotoxic activities of leaves, twigs and stem bark of Scutia buxifolia Reissek. Nat Prod Res. 2012;26:939–944. doi: 10.1080/14786419.2010.535151. [DOI] [PubMed] [Google Scholar]
- 28.Boligon A.A., Kubiça T.F., Mario D.N., Brum T.F., Piana M., Weiblen R. Antimicrobial and antiviral activity-guided fractionation from Scutia buxifolia Reissek extracts. Acta Physiol Plantarum. 2013;35:2229–2239. [Google Scholar]
- 29.de Freitas R.B., Rovani B.T., Boligon A.A., de Brum T.F., Piana M., da Silva J.R. Hepatotoxicity evaluation of aqueous extract from Scutia buxifolia. Molecules. 2013;18:7570–7583. doi: 10.3390/molecules18077570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 31.Woisky R.G., Salatino A. Analysis of própolis: some parameters and procedures for chemical quality control. J Apic Res. 1998;37:99–105. [Google Scholar]
- 32.Morrison M., Asiedu E.A., Stuchbury T., Powell A.A. Determination of lignin and tannin contents of cowpea seeds coats. Ann Bot. 1995;76:287–290. [Google Scholar]
- 33.Sreevidja N., Mehrotra S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff׳s reagent in plant materials. J AOAC Int. 2003;86:1124–1127. [PubMed] [Google Scholar]
- 34.Sabir S.M., Ahmad S.D., Hamid A., Khan M.Q., Athayde M.L., Santos D.B. Antioxidant and hepatoprotective activity of ethanolic extract of leaves of Solidago microglossa containing polyphenolic compounds. Food Chem. 2012;131:741–747. [Google Scholar]
- 35.Ineu R.P., Oliveira C.S., Oliveira V.A., Moraes-Silva L., Luz S.C.A., Pereira M.E. Antioxidant effect of zinc chloride against ethanol-induced gastrointestinal lesions in rats. Food Chem Toxicol. 2013;58:522–529. doi: 10.1016/j.fct.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Ohta Y., Kobayashi T., Nishida K., Ishiguro I. Relationship between changes of active oxygen metabolism and blood flow and formation, progression, and recovery of lesions in gastric mucosa of rats with a single treatment of compound 48/80, a mast cell degranulator. Dig Dis Sci. 1997;42:1221–1232. doi: 10.1023/a:1018854107623. [DOI] [PubMed] [Google Scholar]
- 37.Yam M.F., Ang L.F., Salmanm I.M., Ameerm O.Z., Lim V., Ong L.M. Orthosiphon stamineus leaf extract protects against ethanol-induced gastropathy in rats. J Med Food. 2009;12:1089–1097. doi: 10.1089/jmf.2008.0005. [DOI] [PubMed] [Google Scholar]
- 38.Robert A., Nezamis J.E., Lancaster C., Hauchar A.J. Cytoprotection by prostaglandins in rats: prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl and thermal injury. Gastroenterology. 1979;77:433–443. [PubMed] [Google Scholar]
- 39.Vollmer R.T. Theory and practice of histological techniques. J Am Med Assoc. 1977;238:2730–2738. [Google Scholar]
- 40.Ohkawa H., Ohishi N., Yagy K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 41.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 42.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 43.Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide: general properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:263–275. [PubMed] [Google Scholar]
- 45.Bala I., Bhardwaj V., Hariharan S., Kumar M.N. Analytical methods for assay of ellagic acid and its solubility studies. J Pharm Biomed Anal. 2006;40:206–210. doi: 10.1016/j.jpba.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Barros M.P., Lemos M., Maistro E.L., Leite M.F., Sousa J.P., Bastos J.K. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Própolis. J Ethnopharmacol. 2008;120:372–377. doi: 10.1016/j.jep.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Alimi H., Hfaiedh N., Bouoni Z., Hfaiedh M., Sakly M., Zourgui L. Antioxidant and antiulcerogenic activities of Opuntia ficus indica f. inermis root extract in rats. Phytomedicine. 2010;17:1120–1126. doi: 10.1016/j.phymed.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Dordevic S., Petrović S., Dobrić S., Milenković M., Vucićević D., Zizić S. Antimicrobial, anti-inflammatory, anti-ulcer and antioxidant activities of Carlina acanthifolia root essential oil. J Ethnopharmacol. 2007;109:458–463. doi: 10.1016/j.jep.2006.08.021. [DOI] [PubMed] [Google Scholar]