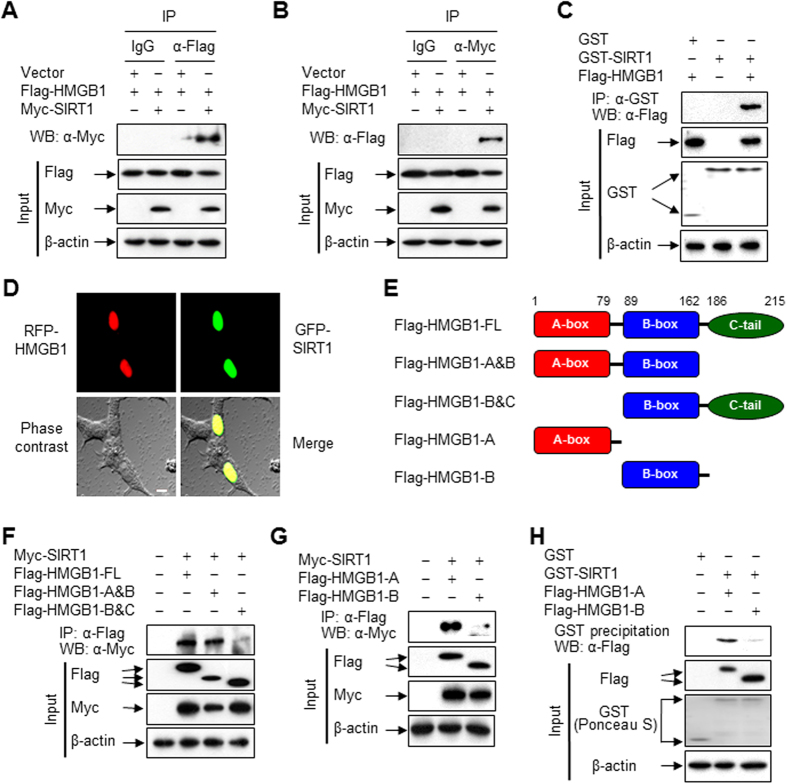
Figure 1. HMGB1 directly interacts with SIRT1.
(A,B) HEK293T cells were co-transfected with the indicated plasmids for 48 h, and then whole-cell lysates were prepared and immunoprecipitated with IgG, anti-Flag, or anti-Myc antibody. The immunoprecipitates and total lysates (input) were subjected to immunoblot analysis with anti-Flag, anti-Myc, and anti-β-actin antibodies to detect HMGB1, SIRT1, and β-actin, respectively. Two percent of whole-cell lysates were used as the input. (C) HEK293T cells were transfected with Flag-tagged HMGB1 for 48 h, and whole-cell lysates were incubated with recombinant GST or GST-SIRT1 fusion protein immobilized to glutathione-Sepharose 4B beads for 20 h. Bead-bound proteins were analyzed by Western blotting. GST and GST-fused proteins were stained with Ponceau S. (D) The fluorescence of each fusion protein was visualized in HEK293T cells by confocal microscopy. The co-localization of HMGB1 and SIRT1 is indicated by the presence of yellow in the merge image. The bar indicates 10 μm. (E) Constructs of Flag-tagged HMGB1 are schematically illustrated. (F,G) HEK293T cells were co-transfected with Myc-SIRT1 and plasmids harboring Flag-HMGB1 FL or deletion mutants for 48 h. Whole-cell lysates were immunoprecipitated with an anti-Flag antibody. (H) HEK293T cells were transfected with Flag-tagged HMGB1 deletion mutants for 48 h. Whole-cell lysates were then prepared and incubated with recombinant GST or GST-SIRT1 fusion protein immobilized to glutathione-Sepharose 4B beads for 20 h. Bead-bound proteins were analyzed by Western blotting. GST and GST-fused proteins were stained with Ponceau S.